Novel viruses of the family Partitiviridae discovered in Saccharomyces cerevisiae
- PMID: 37285383
- PMCID: PMC10281585
- DOI: 10.1371/journal.ppat.1011418
Novel viruses of the family Partitiviridae discovered in Saccharomyces cerevisiae
Abstract
It has been 49 years since the last discovery of a new virus family in the model yeast Saccharomyces cerevisiae. A large-scale screen to determine the diversity of double-stranded RNA (dsRNA) viruses in S. cerevisiae has identified multiple novel viruses from the family Partitiviridae that have been previously shown to infect plants, fungi, protozoans, and insects. Most S. cerevisiae partitiviruses (ScPVs) are associated with strains of yeasts isolated from coffee and cacao beans. The presence of partitiviruses was confirmed by sequencing the viral dsRNAs and purifying and visualizing isometric, non-enveloped viral particles. ScPVs have a typical bipartite genome encoding an RNA-dependent RNA polymerase (RdRP) and a coat protein (CP). Phylogenetic analysis of ScPVs identified three species of ScPV, which are most closely related to viruses of the genus Cryspovirus from the mammalian pathogenic protozoan Cryptosporidium parvum. Molecular modeling of the ScPV RdRP revealed a conserved tertiary structure and catalytic site organization when compared to the RdRPs of the Picornaviridae. The ScPV CP is the smallest so far identified in the Partitiviridae and has structural homology with the CP of other partitiviruses but likely lacks a protrusion domain that is a conspicuous feature of other partitivirus particles. ScPVs were stably maintained during laboratory growth and were successfully transferred to haploid progeny after sporulation, which provides future opportunities to study partitivirus-host interactions using the powerful genetic tools available for the model organism S. cerevisiae.
Copyright: © 2023 Taggart et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


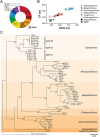


References
-
- Hollings M. Viruses Associated with A Die-Back Disease of Cultivated Mushroom. Nature. 1962;196: 962–965. doi: 10.1038/196962a0 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

