Regorafenib in patients with relapsed advanced or metastatic chordoma: results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre phase II study
- PMID: 37285716
- PMCID: PMC10265601
- DOI: 10.1016/j.esmoop.2023.101569
Regorafenib in patients with relapsed advanced or metastatic chordoma: results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre phase II study
Abstract
Background: REGOBONE multicohort study explored the efficacy and safety of regorafenib for patients with advanced bone sarcomas; this report details the cohort of patients with relapsed advanced or metastatic chordoma.
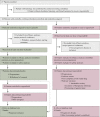
Methods: Patients with relapsed chordoma progressing despite 0-2 prior lines of systemic therapy, were randomised (2 : 1) to receive regorafenib (160 mg/day, 21/28 days) or placebo. Patients on placebo could cross over to receive regorafenib after centrally-confirmed progression. The primary endpoint was the progression-free rate at 6 months (PFR-6) (by RECIST 1.1). With one-sided α of 0.05, and 80% power, at least 10/24 progression-free patients at 6 months (PFR-6) were needed for success.
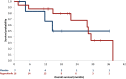
Results: From March 2016 to February 2020, 27 patients were enrolled. A total of 23 patients were assessable for efficacy: 7 on placebo, 16 on regorafenib, 16 were men, median age was 66 (32-85) years. At 6 months, in the regorafenib arm, 1 patient was not assessable, 6/14 were non-progressive (PFR-6: 42.9%; one-sided 95% CI = 20.6) 3/14 discontinued regorafenib due to toxicity; and in the placebo arm, 2/5 patients were non-progressive (PFR-6: 40.0%; one-sided 95% CI = 7.6), 2 were non-assessable. Median progression-free survival was 8.2 months (95% CI 4.5-12.9 months) on regorafenib and 10.1 months (95% CI 0.8 months-non evaluable [NE]) on placebo. Median overall survival rates were 28.3 months (95% CI 14.8 months-NE) on regorafenib but not reached in placebo arm. Four placebo patients crossed over to receive regorafenib after centrally-confirmed progression. The most common grade ≥3 regorafenib-related adverse events were hand-foot skin reaction (22%), hypertension (22%), pain (22%), and diarrhoea (17%), with no toxic death.
Conclusion: This study failed to show any signal of benefit for regorafenib in patients with advanced/metastatic recurrent chordoma.
Trial registration: ClinicalTrials.gov 02389244.
Keywords: recurrent/metastatic chordoma; regorafenib.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
FD received travel grants from Pharmamar, Leo Pharma, attended advisory boards for Bayer, Lilly, Pharmamar, GlaxoSmithKline, Deciphera. All other authors have declared no conflicts of interest.
Figures
References
-
- Strauss S.J., Frezza A.M., Abecassis N., et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–1536. - PubMed
-
- Casali P.G., Stacchiotti S., Sangalli C., Olmi P., Gronchi A. Chordoma. Curr Opin Oncol. 2007;19:367–370. - PubMed
-
- DuBois S.G., Shusterman S., Ingle A.M., et al. A pediatric phase I trial and pharmacokinetic (PK) study of sunitinib: a Children’s Oncology group Phase I Consortium trial. J Clin Oncol. 2008;26(suppl 15):3561.
-
- Pacey S., Ratain M.J., Flaherty M., et al. Efficacy and safety of sorafenib in a subset of patients with soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs. 2009;29(3):481–488. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources