Obesity and renal cell carcinoma: Biological mechanisms and perspectives
- PMID: 37286114
- PMCID: PMC10526958
- DOI: 10.1016/j.semcancer.2023.06.001
Obesity and renal cell carcinoma: Biological mechanisms and perspectives
Abstract
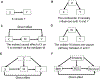
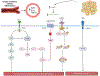
Obesity, defined by body mass index (BMI), is an established risk factor for specific renal cell carcinoma (RCC) subtypes such as clear cell RCC, the most common RCC histology. Many studies have identified an association between obesity and improved survival after diagnosis of RCC, a potential "obesity paradox." Clinically, there is uncertainty whether improved outcomes observed after diagnosis are driven by stage, type of treatment received, or artifacts of longitudinal changes in weight and body composition. The biological mechanisms underlying obesity's influence on RCC are not fully established, but multiomic and mechanistic studies suggest an impact on tumor metabolism, particularly fatty acid metabolism, angiogenesis, and peritumoral inflammation, which are known to be key biological hallmarks of clear cell RCC. Conversely, high-intensity exercise associated with increased muscle mass may be a risk factor for renal medullary carcinoma, a rare RCC subtype that predominantly occurs in individuals with sickle hemoglobinopathies. Herein, we highlight methodologic challenges associated with studying the influence of obesity on RCC and review the clinical evidence and potential underlying mechanisms associating RCC with BMI and body composition.
Keywords: Angiogenesis; Body composition; Fatty acid; Metabolism; Obesity; Renal cell carcinoma.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest NV has no conflicts of interest to disclose. AM reports ownership of equities in Oltre Medical Consulting, LLC (Toulouse, France). AWH reports advisory board consulting to Janssen and Intellisphere and travel support from Dava Oncology. JLM reports advisory board consulting for Bristol Myers Squibb, Roche, and Merck. PM reports honoraria for service on a scientific advisory board for Mirati Therapeutics, Bristol Myers Squibb, and Exelixis; consulting for Axiom Healthcare Strategies; non-branded educational programs supported by Exelixis and Pfizer; and research funding for clinical trials from Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center.
Figures



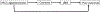
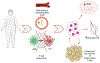
Similar articles
-
Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study.Lancet Oncol. 2020 Feb;21(2):283-293. doi: 10.1016/S1470-2045(19)30797-1. Epub 2019 Dec 20. Lancet Oncol. 2020. PMID: 31870811 Free PMC article.
-
Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies.BJU Int. 2010 Jan;105(1):16-20. doi: 10.1111/j.1464-410X.2009.08706.x. Epub 2009 Jul 6. BJU Int. 2010. PMID: 19583732 Free PMC article.
-
The role of obesity and weight fluctuations in the etiology of renal cell cancer: a population-based case-control study.Cancer Epidemiol Biomarkers Prev. 1994 Dec;3(8):631-9. Cancer Epidemiol Biomarkers Prev. 1994. PMID: 7881335
-
Renal cell carcinoma (RCC): fatter is better? A review on the role of obesity in RCC.Endocr Relat Cancer. 2021 Jun 2;28(7):R207-R216. doi: 10.1530/ERC-20-0457. Endocr Relat Cancer. 2021. PMID: 33949971 Review.
-
Effect of smoking, hypertension and lifestyle factors on kidney cancer - perspectives for prevention and screening programmes.Nat Rev Urol. 2023 Nov;20(11):669-681. doi: 10.1038/s41585-023-00781-8. Epub 2023 Jun 16. Nat Rev Urol. 2023. PMID: 37328546 Review.
Cited by
-
Global Burden of Kidney Cancer Attributable to High Body Mass Index in Adults Aged 60 and Older from 1990 to 2021 and Projections to 2040: A Systematic Analysis for the Global Burden of Disease Study.Clin Epidemiol. 2025 May 21;17:453-479. doi: 10.2147/CLEP.S521272. eCollection 2025. Clin Epidemiol. 2025. PMID: 40417134 Free PMC article.
-
Treatment Outcomes in Patients With Metastatic Renal Cell Carcinoma With Sarcomatoid and/or Rhabdoid Dedifferentiation After Progression on Immune Checkpoint Therapy.Oncologist. 2024 May 3;29(5):392-399. doi: 10.1093/oncolo/oyad302. Oncologist. 2024. PMID: 38035767 Free PMC article.
-
Association of body mass index with clinicopathological features among patients with clear cell renal cell carcinoma treated with surgery: a retrospective study.Sci Rep. 2025 Jan 2;15(1):432. doi: 10.1038/s41598-024-84684-7. Sci Rep. 2025. PMID: 39748015 Free PMC article.
-
Cardiometabolic comorbidities and complications of obesity and chronic kidney disease (CKD).J Clin Transl Endocrinol. 2024 Apr 2;36:100341. doi: 10.1016/j.jcte.2024.100341. eCollection 2024 Jun. J Clin Transl Endocrinol. 2024. PMID: 38616864 Free PMC article. Review.
-
Predicting the prognosis of patients with renal cell carcinoma based on the systemic immune inflammation index and prognostic nutritional index.Sci Rep. 2024 Oct 23;14(1):25045. doi: 10.1038/s41598-024-76519-2. Sci Rep. 2024. PMID: 39443568 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Moch H, Amin MB, Berney DM, et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2022;82:458–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

