Vaccinating against cancer: getting to prime time
- PMID: 37286302
- PMCID: PMC10254974
- DOI: 10.1136/jitc-2022-006628
Vaccinating against cancer: getting to prime time
Abstract
Immunotherapies, such as immune checkpoint inhibitors, cellular therapies, and T-cell engagers, have fundamentally changed our approach to treating cancer. However, successes with cancer vaccines have been more difficult to realize. While vaccines against specific viruses have been widely adopted to prevent the development of cancer, only two vaccines can improve survival in advanced disease: sipuleucel-T and talimogene laherparepvec. These represent the two approaches that have the most traction: vaccinating against cognate antigen and priming responses using tumors in situ. Here, we review the challenges and opportunities researchers face in developing therapeutic vaccines for cancer.
Keywords: Adjuvants, Immunologic; Antigens, Neoplasm; Immunity; Vaccination.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LF has received research support from Roche/Genentech, Abbvie, Bavarian Nordic, Bristol Myers Squibb, Dendreon, Janssen, Merck, and Partner Therapeutics. LF has served on the scientific advisory boards of Actym, Allector, Astra Zeneca, Atreca, Bioalta, Bolt, Bristol Myer Squibb, Daiichi Sankyo, Immunogenesis, Innovent, Merck, Merck KGA, Nutcracker, RAPT, Scribe, Senti, Soteria, Sutro, and Roche/Genentech.
Figures

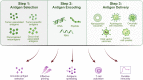
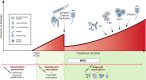
References
-
- Cancer Research Institute . PD-1/PD-L1 landscape. Available: https://www.cancerresearch.org/pd-1-pd-l1-landscape [Accessed 21 Mar 2023].
-
- US Food and Drug Administration . BCG LIVE (for intravesical use). Organon package insert. Available: https://www.fda.gov/media/76396/download [Accessed 21 Mar 2023].
-
- Lamm DL, Blumenstein BA, David Crawford E, et al. . Randomized Intergroup comparison of Bacillus Calmette-Guerin Immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a Southwest oncology group study. Urol Oncol 1995;1:119–26. 10.1016/1078-1439(95)00041-f - DOI - PubMed
