Tebentafusp in combination with durvalumab and/or tremelimumab in patients with metastatic cutaneous melanoma: a phase 1 study
- PMID: 37286303
- PMCID: PMC10254987
- DOI: 10.1136/jitc-2023-006747
Tebentafusp in combination with durvalumab and/or tremelimumab in patients with metastatic cutaneous melanoma: a phase 1 study
Abstract
Background: Immune checkpoint inhibitors have significantly improved outcomes in first line cutaneous melanoma. However, there is a high unmet need for patients who progress on these therapies and combination therapies are being explored to improve outcomes. Tebentafusp is a first-in-class gp100×CD3 ImmTAC bispecific that demonstrated overall survival (OS) benefit (HR 0.51) in metastatic uveal melanoma despite a modest overall response rate of 9%. This phase 1b trial evaluated the safety and initial efficacy of tebentafusp in combination with durvalumab (anti-programmed death ligand 1 (PDL1)) and/or tremelimumab (anti-cytotoxic T lymphocyte-associated antigen 4) in patients with metastatic cutaneous melanoma (mCM), the majority of whom progressed on prior checkpoint inhibitors.
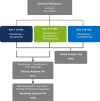
Methods: In this open-label, multicenter, phase 1b, dose-escalation trial, HLA-A*02:01-positive patients with mCM received weekly intravenous tebentafusp with increasing monthly doses of durvalumab and/or tremelimumab starting day 15 of each cycle. The primary objective was to identify the maximum tolerated dose (MTD) or recommended phase 2 dose for each combination. Efficacy analyses were performed in all tebentafusp with durvalumab±tremelimumab treated patients with a sensitivity analysis in those who progressed on prior anti-PD(L)1 therapy.
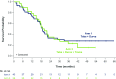
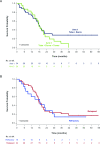
Results: 85 patients were assigned to receive tebentafusp in combination with durvalumab (n=43), tremelimumab (n=13), or durvalumab and tremelimumab (n=29). Patients were heavily pretreated with a median of 3 prior lines of therapy, including 76 (89%) who received prior anti-PD(L)1. Maximum target doses of tebentafusp (68 mcg) alone or in combination with durvalumab (20 mg/kg) and tremelimumab (1 mg/kg) were tolerated; MTD was not formally identified for any arm. Adverse event profile was consistent with each individual therapy and there were no new safety signals nor treatment-related deaths. In the efficacy subset (n=72), the response rate was 14%, tumor shrinkage rate was 41% and 1-year OS rate was 76% (95% CI: 70% to 81%). The 1-year OS for triplet combination (79%; 95% CI: 71% to 86%) was similar to tebentafusp plus durvalumab (74%; 95% CI: 67% to 80%).
Conclusion: At maximum target doses, the safety of tebentafusp with checkpoint inhibitors was consistent with safety of each individual therapy. Tebentafusp with durvalumab demonstrated promising efficacy in heavily pretreated patients with mCM, including those who progressed on prior anti-PD(L)1.
Trial registration number: NCT02535078.
Keywords: Immune Checkpoint Inhibitors; Immunotherapy; Melanoma; T-Lymphocytes.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Figures

Similar articles
-
Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial.JAMA Oncol. 2020 May 1;6(5):661-674. doi: 10.1001/jamaoncol.2020.0237. JAMA Oncol. 2020. PMID: 32271377 Free PMC article. Clinical Trial.
-
Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial.Lancet Oncol. 2020 Dec;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6. Epub 2020 Sep 21. Lancet Oncol. 2020. PMID: 32971005 Clinical Trial.
-
Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial.Lancet Oncol. 2022 Feb;23(2):279-291. doi: 10.1016/S1470-2045(21)00658-6. Epub 2022 Jan 13. Lancet Oncol. 2022. PMID: 35033226 Free PMC article. Clinical Trial.
-
Is tebentafusp superior to combined immune checkpoint blockade and other systemic treatments in metastatic uveal melanoma? A comparative efficacy analysis with population adjustment.Cancer Treat Rev. 2023 Apr;115:102543. doi: 10.1016/j.ctrv.2023.102543. Epub 2023 Mar 13. Cancer Treat Rev. 2023. PMID: 36931146
-
Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma.Cancers (Basel). 2019 Jul 11;11(7):971. doi: 10.3390/cancers11070971. Cancers (Basel). 2019. PMID: 31336704 Free PMC article. Review.
Cited by
-
Immuno-oncology approaches in uveal melanoma: tebentafusp and beyond.Immunooncol Technol. 2023 Jun 8;19:100386. doi: 10.1016/j.iotech.2023.100386. eCollection 2023 Sep. Immunooncol Technol. 2023. PMID: 37483658 Free PMC article. Review.
-
Efficacy and safety of tebentafusp in patients with metastatic uveal melanoma: A systematic review and meta-analysis.Hum Vaccin Immunother. 2024 Dec 31;20(1):2374647. doi: 10.1080/21645515.2024.2374647. Epub 2024 Jul 14. Hum Vaccin Immunother. 2024. PMID: 39004419 Free PMC article.
-
Gold nanostructures in melanoma: Advances in treatment, diagnosis, and theranostic applications.Heliyon. 2024 Aug 2;10(15):e35655. doi: 10.1016/j.heliyon.2024.e35655. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170173 Free PMC article. Review.
-
Immunotherapy in melanoma: advances, pitfalls, and future perspectives.Front Mol Biosci. 2024 Jun 28;11:1403021. doi: 10.3389/fmolb.2024.1403021. eCollection 2024. Front Mol Biosci. 2024. PMID: 39086722 Free PMC article. Review.
-
Clinical benefit with tebentafusp in a patient with GNAQ mutant metastatic blue nevus-associated melanoma.J Immunother Cancer. 2024 Nov 17;12(11):e009609. doi: 10.1136/jitc-2024-009609. J Immunother Cancer. 2024. PMID: 39551602 Free PMC article.
References
-
- SEER cancer STAT facts: Melanoma of the skin [Internet]. 2022. Available: https://seer.cancer.gov/statfacts/html/melan.html#ref11
-
- Feigelson HS, Powers JD, Kumar M, et al. . Melanoma incidence, recurrence, and mortality in an integrated Healthcare system: A retrospective cohort study. Cancer Med 2019;8:4508–16. 10.1002/cam4.2252 Available: https://onlinelibrary.wiley.com/toc/20457634/8/9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
