Bacteria-phage coevolution with a seed bank
- PMID: 37286738
- PMCID: PMC10356755
- DOI: 10.1038/s41396-023-01449-2
Bacteria-phage coevolution with a seed bank
Abstract
Dormancy is an adaptation to living in fluctuating environments. It allows individuals to enter a reversible state of reduced metabolic activity when challenged by unfavorable conditions. Dormancy can also influence species interactions by providing organisms with a refuge from predators and parasites. Here we test the hypothesis that, by generating a seed bank of protected individuals, dormancy can modify the patterns and processes of antagonistic coevolution. We conducted a factorially designed experiment where we passaged a bacterial host (Bacillus subtilis) and its phage (SPO1) in the presence versus absence of a seed bank consisting of dormant endospores. Owing in part to the inability of phages to attach to spores, seed banks stabilized population dynamics and resulted in minimum host densities that were 30-fold higher compared to bacteria that were unable to engage in dormancy. By supplying a refuge to phage-sensitive strains, we show that seed banks retained phenotypic diversity that was otherwise lost to selection. Dormancy also stored genetic diversity. After characterizing allelic variation with pooled population sequencing, we found that seed banks retained twice as many host genes with mutations, whether phages were present or not. Based on mutational trajectories over the course of the experiment, we demonstrate that seed banks can dampen bacteria-phage coevolution. Not only does dormancy create structure and memory that buffers populations against environmental fluctuations, it also modifies species interactions in ways that can feed back onto the eco-evolutionary dynamics of microbial communities.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


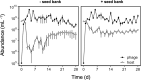
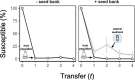
 ). In addition, we revived clones (n = 22) from the external seed bank (Fig. 1) at each time point and challenged those against the ancestral phage (
). In addition, we revived clones (n = 22) from the external seed bank (Fig. 1) at each time point and challenged those against the ancestral phage ( ). The expected percentage of susceptible clones (
). The expected percentage of susceptible clones ( ) is based on losses to dilution caused by serial transfer of clones originating from the pre-infection external seed bank (see Supplementary Information). Data represented as mean ± SEM of the replicate populations (n = 3).
) is based on losses to dilution caused by serial transfer of clones originating from the pre-infection external seed bank (see Supplementary Information). Data represented as mean ± SEM of the replicate populations (n = 3).

References
-
- Thompson JN. The Coevolutionary Process. University of Chicago Press; Chicago, 1994.
-
- Allen RL, Bittner-Eddy PD, Grenville-Briggs LJ, Meitz JC, Rehmany AP, Rose LE, et al. Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science. 2004;306:1957–60. - PubMed

