Meaningful Within-Patient Change on the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ): Analysis of Phase III Clinical Trial Data of Daridorexant
- PMID: 37286927
- PMCID: PMC10314845
- DOI: 10.1007/s40290-023-00484-w
Meaningful Within-Patient Change on the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ): Analysis of Phase III Clinical Trial Data of Daridorexant
Abstract
Background: The Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ) is a new validated 14-item patient-reported outcome (PRO) instrument for evaluating daytime functioning in people with insomnia. It comprises three domains: Alert/Cognition, Mood, and Sleepiness.
Objective: The aim of this analysis was to estimate the minimum within-patient change for IDSIQ scores that an adult patient with insomnia would consider meaningful.
Methods: Data were from a randomized, double-blind, placebo-controlled, phase III clinical trial of daridorexant in adults with insomnia. Subjects completed the IDSIQ daily in the evening, with a recall period of 'today', throughout the 3-month double-blind treatment period. Scores were calculated as a weekly average. Each IDSIQ item was scored on an 11-point numeric rating scale ranging from 0 (not at all/none at all) to 10 (very/a lot), with a higher score indicating a greater severity or impact. PRO measures with correlation coefficients ≥0.30 were included in a subsequent anchor-based analysis. For the IDSIQ total score and each IDSIQ domain, meaningful within-patient change was estimated as the minimum score change patients would consider meaningful in an anchor-based analysis using data from PRO instruments capturing daytime and night-time insomnia symptoms (the Insomnia Severity Index [four items, each scored 0-4, with a higher score indicating greater symptom severity; assessed at screening, baseline, month 1 and month 3], Patient Global Assessment of Disease Severity [6-point scale from 'none' to 'very severe'; assessed weekly], Patient Global Impression of Severity [4-point scale from 'none' to 'severe'; assessed weekly], and Patient Global Impression of Change [7-point scale from 'very much better' to 'very much worse'; assessed weekly for night-time and daytime symptoms separately]). A supplemental distribution-based analysis was also conducted to support the anchor-based analysis.
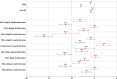
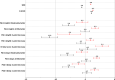
Results: The analysis included 930 subjects aged 18-88 years. Spearman correlation coefficients for the relationships between score changes/ratings for anchors and the IDSIQ (0.36-0.44 at month 1, 0.45-0.57 at month 3) were all above the prespecified threshold of 0.30. Mean IDSIQ score changes at months 1 and 3 based on the different anchors supported meaningful within-patient change estimates starting at 17 points for the IDSIQ total score, 9 points for the Alert/Cognition domain, and 4 points for the Mood and Sleepiness domains.
Conclusion: This analysis demonstrates the meaningful within-patient change for the IDSIQ total score and domain scores, that the instrument is sensitive to changes in the patient experience of insomnia, and that it can be used in clinical trials to evaluate changes in daytime functioning.
Clinical trials registration: NCT03545191 (4 June 2018).
© 2023. The Author(s).
Conflict of interest statement
Andrea Phillips-Beyer is the director of Innovus Consulting Ltd, which received financial support from Idorsia for work on this study. Ariane K. Kawata and Leah Kleinman are employees of Evidera, which received financial support from Idorsia (via Innovus) for work on this study. Dalma Seboek Kinter is an employee of Idorsia.
Figures


References
-
- US FDA. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/media/77832/download. Accessed 23 May 2023. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

