Role of high-temperature requirement serine protease A 2 in rheumatoid inflammation
- PMID: 37287073
- PMCID: PMC10246393
- DOI: 10.1186/s13075-023-03081-z
Role of high-temperature requirement serine protease A 2 in rheumatoid inflammation
Abstract
Background: High-temperature requirement serine protease A 2 (HtrA2) is known to be involved in growth, unfolded protein response to stress, apoptosis, and autophagy. However, whether HtrA2 controls inflammation and immune response remains elusive.
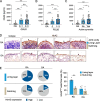
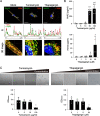
Methods: Expression of HtrA2 in the synovial tissue of patients was examined using immunohistochemistry and immunofluorescence staining. Enzyme-linked immunosorbent assay was used to determine the concentrations of HtrA2, interleukin-6 (IL-6), interleukin-8 (IL-8), chemokine (C-C motif) ligand 2 (CCL2), and tumor necrosis factor α (TNFα). Synoviocyte survival was assessed by MTT assay. For the downregulation of HtrA2 transcripts, cells were transfected with HtrA2 siRNA.
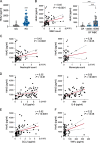
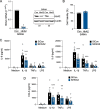
Results: We found that the concentration of HtrA2 was elevated in rheumatoid arthritis (RA) synovial fluid (SF) than in osteoarthritis (OA) SF, and its concentrations were correlated with the number of immune cells in the RA SF. Interestingly, HtrA2 levels in the SF of RA patients were elevated in proportion to synovitis severity and correlated with the expression of proinflammation cytokines and chemokines, such as IL-6, IL-8, and CCL2. In addition, HtrA2 was highly expressed in RA synovium and primary synoviocytes. RA synoviocytes released HtrA2 when stimulated with ER stress inducers. Knockdown of HtrA2 inhibited the IL1β-, TNFα-, and LPS-induced release of proinflammatory cytokines and chemokines by RA synoviocytes.
Conclusion: HtrA2 is a novel inflammatory mediator and a potential target for the development of an anti-inflammation therapy for RA.
Keywords: Fibroblast-like synoviocytes; High-temperature requirement serine protease A; Inflammation; Rheumatoid arthritis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

