Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway
- PMID: 37287079
- PMCID: PMC10249217
- DOI: 10.1186/s13287-023-03387-4
Endometrium-derived mesenchymal stem cells suppress progression of endometrial cancer via the DKK1-Wnt/β-catenin signaling pathway
Abstract
Background: Mesenchymal stem cell (MSC) therapy is an attractive treatment option for various cancers. Whether MSCs can be used to treat well-differentiated endometrial cancer (EC) remains unclear. The aim of this study is to explore the potential therapeutic effects of MSCs on EC and the underlying mechanisms.
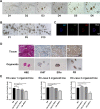
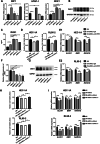
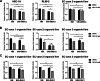
Methods: The effects of adipose-derived MSCs (AD-MSCs), umbilical-cord-derived MSCs (UC-MSCs), and endometrium-derived MSCs (eMSCs) on the malignant behaviors of EC cells were explored via in vitro and in vivo experiments. Three EC models, including patient-derived EC organoid lines, EC cell lines, and EC xenograft model in female BALB/C nude mice, were used for this study. The effects of MSCs on EC cell proliferation, apoptosis, migration, and the growth of xenograft tumors were evaluated. The potential mechanisms by which eMSCs inhibit EC cell proliferation and stemness were explored by regulating DKK1 expression in eMSCs or Wnt signaling in EC cells.
Results: Our results showed that eMSCs had the highest inhibitory effect on EC cell viability, and EC xenograft tumor growth in mice compared to AD-MSCs and UC-MSCs. Conditioned medium (CM) obtained from eMSCs significantly suppressed the sphere-forming ability and stemness-related gene expression of EC cells. In comparison to AD-MSCs and UC-MSCs, eMSCs had the highest level of Dickkopf-related protein 1 (DKK1) secretion. Mechanistically, eMSCs inhibited Wnt/β-catenin signaling in EC cells via secretion of DKK1, and eMSCs suppressed EC cell viability and stemness through DKK1-Wnt/β-catenin signaling. Additionally, the combination of eMSCs and medroxyprogesterone acetate (MPA) significantly inhibited the viability of EC organoids and EC cells compared with eMSCs or MPA alone.
Conclusions: The eMSCs, but not AD-MSCs or UC-MSCs, could suppress the malignant behaviors of EC both in vivo and in vitro via inhibiting the Wnt/β-catenin signaling pathway by secreting DKK1. The combination of eMSCs and MPA effectively inhibited EC growth, indicating that eMSCs may potentially be a new therapeutic strategy for young EC patients desiring for fertility preservation.
Keywords: DKK1; Endometrial cancer; Wnt/β-catenin signaling; eMSCs.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures




Similar articles
-
Myometrial Cells Stimulate Self-Renewal of Endometrial Mesenchymal Stem-Like Cells Through WNT5A/β-Catenin Signaling.Stem Cells. 2019 Nov;37(11):1455-1466. doi: 10.1002/stem.3070. Epub 2019 Oct 8. Stem Cells. 2019. PMID: 31414525
-
Human umbilical cord mesenchymal stem cells inhibit C6 glioma growth via secretion of dickkopf-1 (DKK1).Mol Cell Biochem. 2014 Jan;385(1-2):277-86. doi: 10.1007/s11010-013-1836-y. Epub 2013 Oct 9. Mol Cell Biochem. 2014. PMID: 24104453
-
DKK1 Activates the PI3K/AKT Pathway via CKAP4 to Balance the Inhibitory Effect on Wnt/β-Catenin Signaling and Regulates Wnt3a-Induced MSC Migration.Stem Cells. 2024 Jun 14;42(6):567-579. doi: 10.1093/stmcls/sxae022. Stem Cells. 2024. PMID: 38469899
-
Perspectives on miRNAs Targeting DKK1 for Developing Hair Regeneration Therapy.Cells. 2021 Oct 30;10(11):2957. doi: 10.3390/cells10112957. Cells. 2021. PMID: 34831180 Free PMC article. Review.
-
Role of non‑coding RNA intertwined with the Wnt/β‑catenin signaling pathway in endometrial cancer (Review).Mol Med Rep. 2023 Aug;28(2):150. doi: 10.3892/mmr.2023.13037. Epub 2023 Jun 23. Mol Med Rep. 2023. PMID: 37350380 Free PMC article. Review.
Cited by
-
Endostatin-expressing endometrial mesenchymal stem cells inhibit angiogenesis in endometriosis through the miRNA-21-5p/TIMP3/PI3K/Akt/mTOR pathway.Stem Cells Transl Med. 2025 Feb 11;14(2):szae079. doi: 10.1093/stcltm/szae079. Stem Cells Transl Med. 2025. PMID: 39589222 Free PMC article.
-
Bioengineering approaches for the endometrial research and application.Mater Today Bio. 2024 Apr 3;26:101045. doi: 10.1016/j.mtbio.2024.101045. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38600921 Free PMC article. Review.
-
The Establishment of 3D Polarity-Reversed Organoids From Human Endometrial Tissue as a Model for Infection-Induced Endometritis.Bio Protoc. 2025 Jun 20;15(12):e5349. doi: 10.21769/BioProtoc.5349. eCollection 2025 Jun 20. Bio Protoc. 2025. PMID: 40620810 Free PMC article.
-
Cancer stem cells: advances in knowledge and implications for cancer therapy.Signal Transduct Target Ther. 2024 Jul 5;9(1):170. doi: 10.1038/s41392-024-01851-y. Signal Transduct Target Ther. 2024. PMID: 38965243 Free PMC article. Review.
-
Exploration of eMSCs with HA-GEL system in repairing damaged endometrium after endometrial cancer with fertility-sparing treatment.Cell Tissue Res. 2023 Nov;394(2):379-392. doi: 10.1007/s00441-023-03831-0. Epub 2023 Sep 28. Cell Tissue Res. 2023. PMID: 37759141
References
-
- Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, Tatebe K, Veneris JL. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

