5-aminolevulinic acid, fluorescein sodium, and indocyanine green for glioma margin detection: analysis of operating wide-field and confocal microscopy in glioma models of various grades
- PMID: 37287908
- PMCID: PMC10242067
- DOI: 10.3389/fonc.2023.1156812
5-aminolevulinic acid, fluorescein sodium, and indocyanine green for glioma margin detection: analysis of operating wide-field and confocal microscopy in glioma models of various grades
Abstract
Introduction: Surgical resection remains the first-line treatment for gliomas. Several fluorescent dyes are currently in use to augment intraoperative tumor visualization, but information on their comparative effectiveness is lacking. We performed systematic assessment of fluorescein sodium (FNa), 5-aminolevulinic acid (5-ALA)-induced protoporphyrin IX (PpIX), and indocyanine green (ICG) fluorescence in various glioma models using advanced fluorescence imaging techniques.
Methods: Four glioma models were used: GL261 (high-grade model), GB3 (low-grade model), and an in utero electroporation model with and without red fluorescence protein (IUE +RFP and IUE -RFP, respectively) (intermediate-to-low-grade model). Animals underwent 5-ALA, FNa, and ICG injections and craniectomy. Brain tissue samples underwent fluorescent imaging using a wide-field operative microscope and a benchtop confocal microscope and were submitted for histologic analysis.
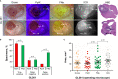
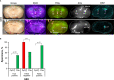
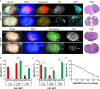
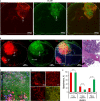
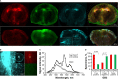
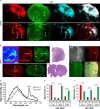
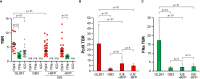
Results: Our systematic analysis showed that wide-field imaging of highly malignant gliomas is equally efficient with 5-ALA, FNa, and ICG, although FNa is associated with more false-positive staining of the normal brain. In low-grade gliomas, wide-field imaging cannot detect ICG staining, can detect FNa in only 50% of specimens, and is not sensitive enough for PpIX detection. With confocal imaging of low-intermediate grade glioma models, PpIX outperformed FNa.
Discussion: Overall, compared to wide-field imaging, confocal microscopy significantly improved diagnostic accuracy and was better at detecting low concentrations of PpIX and FNa, resulting in improved tumor delineation. Neither PpIX, FNa, nor ICG delineated all tumor boundaries in studied tumor models, which emphasizes the need for novel visualization technologies and molecular probes to guide glioma resection. Simultaneous administration of 5-ALA and FNa with use of cellular-resolution imaging modalities may provide additional information for margin detection and may facilitate maximal glioma resection.
Keywords: 5-aminolevulinic acid; fluorescein sodium; fluorescence guided surgery; glioma; indocyanine green; laser scanning microscopy; protoporphyrin IX.
Copyright © 2023 Belykh, Bardonova, Abramov, Byvaltsev, Kerymbayev, Yu, Healey, Luna-Melendez, Deneen, Mehta, Liu and Preul.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Zhao S, Wu J, Wang C, Liu H, Dong X, Shi C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PloS One (2013) 8(5):e63682. doi: 10.1371/journal.pone.0063682 - DOI - PMC - PubMed
-
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol (2006) 7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources

