Simultaneous co-infection with swine influenza A and porcine reproductive and respiratory syndrome viruses potentiates adaptive immune responses
- PMID: 37287962
- PMCID: PMC10242126
- DOI: 10.3389/fimmu.2023.1192604
Simultaneous co-infection with swine influenza A and porcine reproductive and respiratory syndrome viruses potentiates adaptive immune responses
Abstract
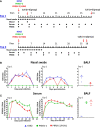
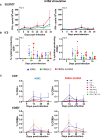
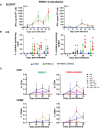
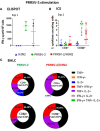
Porcine respiratory disease is multifactorial and most commonly involves pathogen co-infections. Major contributors include swine influenza A (swIAV) and porcine reproductive and respiratory syndrome (PRRSV) viruses. Experimental co-infection studies with these two viruses have shown that clinical outcomes can be exacerbated, but how innate and adaptive immune responses contribute to pathogenesis and pathogen control has not been thoroughly evaluated. We investigated immune responses following experimental simultaneous co-infection of pigs with swIAV H3N2 and PRRSV-2. Our results indicated that clinical disease was not significantly exacerbated, and swIAV H3N2 viral load was reduced in the lung of the co-infected animals. PRRSV-2/swIAV H3N2 co-infection did not impair the development of virus-specific adaptive immune responses. swIAV H3N2-specific IgG serum titers and PRRSV-2-specific CD8β+ T-cell responses in blood were enhanced. Higher proportions of polyfunctional CD8β+ T-cell subset in both blood and lung washes were found in PRRSV-2/swIAV H3N2 co-infected animals compared to the single-infected groups. Our findings provide evidence that systemic and local host immune responses are not negatively affected by simultaneous swIAV H3N2/PRRSV-2 co-infection, raising questions as to the mechanisms involved in disease modulation.
Keywords: immune response; pig; porcine reproductive and respiratory syndrome virus; swine influenza A virus; viral co-infection.
Copyright © 2023 Chrun, Maze, Roper, Vatzia, Paudyal, McNee, Martini, Manjegowda, Freimanis, Silesian, Polo, Clark, Besell, Booth, Carr, Edmans, Nunez, Koonpaew, Wanasen, Graham and Tchilian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
- BB/R01275X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007031/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007037/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

