Functionalisation of conjugated macrocycles with type I and II concealed antiaromaticity via cross-coupling reactions
- PMID: 37288099
- PMCID: PMC10243434
- DOI: 10.1039/d3me00045a
Functionalisation of conjugated macrocycles with type I and II concealed antiaromaticity via cross-coupling reactions
Abstract
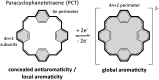
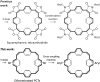
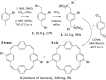
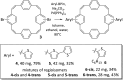
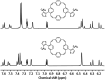
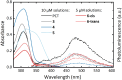
Conjugated macrocycles can exhibit concealed antiaromaticity; that is, despite not being antiaromatic, under specific circumstances, they can display properties typically observed in antiaromatic molecules due to their formal macrocyclic 4n π-electron system. Paracyclophanetetraene (PCT) and its derivatives are prime examples of macrocycles exhibiting this behaviour. In redox reactions and upon photoexcitation, they have been shown to behave like antiaromatic molecules (requiring type I and II concealed antiaromaticity, respectively), with such phenomena showing potential for use in battery electrode materials and other electronic applications. However, further exploration of PCTs has been hindered by the lack of halogenated molecular building blocks that would permit their integration into larger conjugated molecules by cross-coupling reactions. Here, we present two dibrominated PCTs, obtained as a mixture of regioisomers from a three-step synthesis, and demonstrate their functionalisation via Suzuki cross-coupling reactions. Optical, electrochemical, and theoretical studies reveal that aryl substituents can subtly tune the properties and behaviour of PCT, showing that this is a viable strategy in further exploring this promising class of materials.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Huber W. Müllen K. Wennerström O. Angew. Chem., Int. Ed. Engl. 1980;19:624–625. doi: 10.1002/anie.198006241. doi: 10.1002/anie.198006241. - DOI
-
- Müllen K. Unterberg H. Huber W. Wennerström O. Norinder U. Tanner D. Thulin B. J. Am. Chem. Soc. 1984;106:7514–7522. doi: 10.1021/ja00336a035. doi: 10.1021/ja00336a035. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
