Mammalian mitochondrial iron-sulfur cluster biogenesis and transfer and related human diseases
- PMID: 37288145
- PMCID: PMC10235907
- DOI: 10.52601/bpr.2021.200038
Mammalian mitochondrial iron-sulfur cluster biogenesis and transfer and related human diseases
Abstract
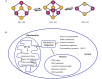
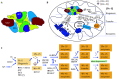
As a cofactor, iron-sulfur (Fe-S) cluster binds to proteins or enzymes that play important roles in various important biological processes, including DNA synthesis and repair, mitochondrial function, gene transcription and translation. In mammals, the core components involved in Fe-S cluster biosynthesis are considered to include the scaffold protein ISCU, cysteine desulfurase NFS1 and its accessory proteins ISD11 and ACP, and frataxin (FXN). Proteins involved in Fe-S cluster transfer have been found to include HSC20/HSPA9, as chaperone system, and Fe-S cluster carriers. The biosynthesis and transfer of Fe-S clusters to Fe-S recipients require fine-tune regulation. Recently, significant progress has been made in the structure and mechanism of mitochondrial Fe-S biosynthesis and transfer. Based on, especially, the development of DNA sequencing technology, bioinformatics, and gene editing technology, diseases caused by mutations of Fe-S cluster-related genes have been revealed in recent years, promoting the rapid development in the field of Fe-S and human health. This review focuses on the function of genes involved in Fe-S cluster biosynthesis and transfer and on the diseases caused by the mutations of the related genes. Finally, some questions we are facing are raised, new hypotheses presented, and the perspectives discussed.
Keywords: Congenital sideroblastic anemia; Fe–S cluster synthesis and transfer; Mitochondria; Neurodegenerative diseases.
© The Author(s) 2021.
Conflict of interest statement
Wenxin Zhang, Li Xu, Hongting Zhao and Kuanyu Li declare that they have no conflict of interest.
Figures
Similar articles
-
Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU).J Biol Chem. 2013 Oct 4;288(40):28755-70. doi: 10.1074/jbc.M113.482042. Epub 2013 Aug 12. J Biol Chem. 2013. PMID: 23940031 Free PMC article.
-
Interactions of iron-bound frataxin with ISCU and ferredoxin on the cysteine desulfurase complex leading to Fe-S cluster assembly.J Inorg Biochem. 2018 Jun;183:107-116. doi: 10.1016/j.jinorgbio.2018.03.007. Epub 2018 Mar 15. J Inorg Biochem. 2018. PMID: 29576242 Free PMC article.
-
Zinc(II) binding on human wild-type ISCU and Met140 variants modulates NFS1 desulfurase activity.Biochimie. 2018 Sep;152:211-218. doi: 10.1016/j.biochi.2018.07.012. Epub 2018 Jul 20. Biochimie. 2018. PMID: 30031876 Free PMC article.
-
Iron-sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery.Biochim Biophys Acta. 2015 Jun;1853(6):1493-512. doi: 10.1016/j.bbamcr.2014.09.009. Epub 2014 Sep 19. Biochim Biophys Acta. 2015. PMID: 25245479 Free PMC article. Review.
-
Molecular characteristics of proteins within the mitochondrial Fe-S cluster assembly complex.Micron. 2022 Feb;153:103181. doi: 10.1016/j.micron.2021.103181. Epub 2021 Nov 12. Micron. 2022. PMID: 34823116 Review.
Cited by
-
ATP-Binding Cassette Transporter of Clinical Significance: Sideroblastic Anemia.J Pers Med. 2024 Jun 14;14(6):636. doi: 10.3390/jpm14060636. J Pers Med. 2024. PMID: 38929857 Free PMC article. Review.
-
The Regulation of the Disease-Causing Gene FXN.Cells. 2024 Jun 15;13(12):1040. doi: 10.3390/cells13121040. Cells. 2024. PMID: 38920668 Free PMC article. Review.
-
Cur@SF NPs alleviate Friedreich's ataxia in a mouse model through synergistic iron chelation and antioxidation.J Nanobiotechnology. 2022 Mar 9;20(1):118. doi: 10.1186/s12951-022-01333-9. J Nanobiotechnology. 2022. PMID: 35264205 Free PMC article.
-
Steroidogenic electron-transfer factors and their diseases.Ann Pediatr Endocrinol Metab. 2021 Sep;26(3):138-148. doi: 10.6065/apem.2142154.077. Epub 2021 Sep 30. Ann Pediatr Endocrinol Metab. 2021. PMID: 34610701 Free PMC article.
-
Disordered Electron Transfer: New Forms of Defective Steroidogenesis and Mitochondriopathy.J Clin Endocrinol Metab. 2025 Feb 18;110(3):e574-e582. doi: 10.1210/clinem/dgae815. J Clin Endocrinol Metab. 2025. PMID: 39574227 Free PMC article. Review.
References
-
- Ajit Bolar N, Vanlander AV, Wilbrecht C, Van der Aa N, Smet J, De Paepe B, Vandeweyer G, Kooy F, Eyskens F, De Latter E, Delanghe G, Govaert P, Leroy JG, Loeys B, Lill R, Van Laer L, Van Coster R Mutation of the iron–sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet. 2013;22(13):2590–2602. doi: 10.1093/hmg/ddt107. - DOI - PubMed
-
- Al-Hassnan ZN, Al-Dosary M, Alfadhel M, Faqeih EA, Alsagob M, Kenana R, Almass R, Al-Harazi OS, Al-Hindi H, Malibari OI, Almutari FB, Tulbah S, Alhadeq F, Al-Sheddi T, Alamro R, AlAsmari A, Almuntashri M, Alshaalan H, Al-Mohanna FA, Colak D, Kaya N ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J Med Genet. 2015;52(3):186–194. doi: 10.1136/jmedgenet-2014-102592. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
