Treating amyloid transthyretin cardiomyopathy: lessons learned from clinical trials
- PMID: 37288260
- PMCID: PMC10242061
- DOI: 10.3389/fcvm.2023.1154594
Treating amyloid transthyretin cardiomyopathy: lessons learned from clinical trials
Abstract
An increasing awareness of the disease, new diagnostic tools and novel therapeutic opportunities have dramatically changed the management of patients with amyloid transthyretin cardiomyopathy (ATTR-CM). Supportive therapies have shown limited benefits, mostly related to diuretics for the relief from signs and symptoms of congestion in patients presenting heart failure (HF). On the other hand, huge advances in specific (disease-modifying) treatments occurred in the last years. Therapies targeting the amyloidogenic cascade include several pharmacological agents that inhibit hepatic synthesis of TTR, stabilize the tetramer, or disrupt fibrils. Tafamidis, a TTR stabilizer that demonstrated to prolong survival and improve quality of life in the ATTR-ACT trial, is currently the only approved drug for patients with ATTR-CM. The small interfering RNA (siRNA) patisiran and the antisense oligonucleotide (ASO) inotersen have been approved for the treatment of patients with hereditary ATTR polyneuropathy regardless of the presence of cardiac involvement, with patisiran also showing preliminary benefits on the cardiac phenotype. Ongoing phase III clinical trials are investigating another siRNA, vutrisiran, and a novel ASO formulation, eplontersen, in patients with ATTR-CM. CRISPR-Cas9 represents a promising strategy of genome editing to obtain a highly effective blockade of TTR gene expression.
Keywords: antisense oligonucleotide; gene editing; heart failure; siRNA; tafamidis; transthyretin cardiac amyloidosis (ATTR-CA); treatment.
© 2023 Tomasoni, Bonfioli, Aimo, Adamo, Canepa, Inciardi, Lombardi, Nardi, Pagnesi, Riccardi, Vergaro, Vizzardi, Emdin and Metra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor (AP) declared a past co-authorship with the authors (DT and MM).
Figures
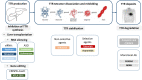
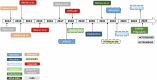
References
-
- Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur J Heart Fail. (2021) 23(4):512–26. 10.1002/ejhf.2140 - DOI - PubMed
-
- Authors/Task Force M, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. 10.1002/ejhf.2333 - DOI - PubMed
-
- Buxbaum JN, Dispenzieri A, Eisenberg DS, Fandrich M, Merlini G, Saraiva MJM, et al. Amyloid nomenclature 2022: update, novel proteins, and recommendations by the international society of amyloidosis (ISA) Nomenclature committee. Amyloid. (2022) 29(4):213–9. 10.1080/13506129.2022.2147636 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

