The versatile role of Serpina3c in physiological and pathological processes: a review of recent studies
- PMID: 37288300
- PMCID: PMC10242157
- DOI: 10.3389/fendo.2023.1189007
The versatile role of Serpina3c in physiological and pathological processes: a review of recent studies
Abstract
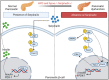
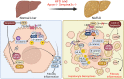
Murine Serpina3c belongs to the family of serine protease inhibitors (Serpins), clade "A" and its human homologue is SerpinA3. Serpina3c is involved in some physiological processes, including insulin secretion and adipogenesis. In the pathophysiological process, the deletion of Serpina3c leads to more severe metabolic disorders, such as aggravated non-alcoholic fatty liver disease (NAFLD), insulin resistance and obesity. In addition, Serpina3c can improve atherosclerosis and regulate cardiac remodeling after myocardial infarction. Many of these processes are directly or indirectly mediated by its inhibition of serine protease activity. Although its function has not been fully revealed, recent studies have shown its potential research value. Here, we aimed to summarize recent studies to provide a clearer view of the biological roles and the underlying mechanisms of Serpina3c.
Keywords: Cardiometabolic diseases; NAFLD; cathepsin G; inflammation; insulin resistance; obesity; serine protease inhibitors; thrombin.
Copyright © 2023 Li and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

