Feasibility of a No-Implant Approach to Interatrial Shunts: Preclinical and Early Clinical Studies
- PMID: 37288335
- PMCID: PMC10242572
- DOI: 10.1016/j.shj.2022.100078
Feasibility of a No-Implant Approach to Interatrial Shunts: Preclinical and Early Clinical Studies
Abstract
Background: Heart failure with preserved ejection fraction represents a major unmet clinical need with limited treatment options. Recent device therapies under investigation have focused on decompression of the left atrium through an implantable interatrial shunt. Although these devices have shown favorable safety and efficacy signals, an implant is required to maintain shunt patency, which may increase the patient risk profile and complicate subsequent interventions requiring transseptal access.
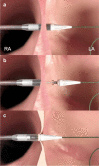
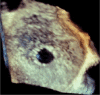
Methods: The Alleviant System is a no-implant approach to creating an interatrial shunt using radiofrequency energy to securely capture, excise, and extract a precise disk of tissue from the interatrial septum. Acute preclinical studies in healthy swine (n = 5) demonstrated the feasibility of the Alleviant System to repeatably create a 7 mm interatrial orifice with minimal collateral thermal effect and minimal platelet and fibrin deposition observed histologically.
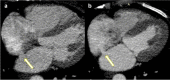
Results: Chronic animal studies (n = 9) were carried out to 30- and 60-day time points and exhibited sustained shunt patency with histology demonstrating completely healed margins, endothelialization, and no trauma to adjacent atrial tissue. Preliminary clinical safety and feasibility were validated in a first-in-human study in patients with heart failure with preserved ejection fraction (n = 15). All patients demonstrated shunt patency by transesophageal echocardiographic imaging at 1, 3, and 6 months, as well as cardiac computed tomography imaging at 6-month follow-up timepoints.
Conclusions: Combined, these data support the safety and feasibility of a novel no-implant approach to creating an interatrial shunt using the Alleviant System. Continued follow-up and subsequent clinical studies are currently ongoing.
Keywords: Heart failure; No-implant shunt; Preserved ejection fraction; Radiofrequency.
© 2022 The Author(s).
Conflict of interest statement
C. M. Barker reports consulting fees from and is an advisory board/board member to Alleviant Medical. C. U. Meduri reports consulting fees from Alleviant Medical, Anteris Technologies, Boston Scientific, Medtronic, Vdyne, speakers’ fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic; he is an advisory board member for Anteris Technologies and Cardiovalve, and a proctor for Boston Scientific. P. S. Fail reports consulting fees from BioVentrix and Alleviant Medical, speakers’ fees from Abbott Vascular, Boston Scientific, and Medtronic, and served as principal investigator of research studies for Ancora Heart and Corvia Medical. J. W. Chambers reports consulting fees from Alleviant Medical and serves as Chief Medical Officer of Cardiovascular Systems Inc. D. J. Solet reports consulting fees from Abbott Medical and Alleviant Medical. J. M. Kriegel reports consulting fees from, is an advisory board/board member to, and serves as Chief Medical Officer of Alleviant Medical. K. Feldt reports consulting fees from Abbott Vascular, Alleviant Medical, Anteris Technologies, Pfizer Inc, and Orion Pharma. T. D. Pate and A. P. Patel are employed by Alleviant Medical. T. Shaburishvili served as principal investigator of a research study for Alleviant Medical. All other authors declare no competing interests.
Figures



References
-
- Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. - PubMed
-
- Shah S.J., Borlaug B.A., Chung E.S., et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399:1130–1140. - PubMed
-
- Paitazoglou C., Bergmann M.W., Ozdemir R., et al. One-year results of the first-in-man study investigating the Atrial Flow Regulator for left atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail. 2021;23:800–810. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

