Redox Dysregulation of Vascular Smooth Muscle Sirtuin-1 in Thoracic Aortic Aneurysm in Marfan Syndrome
- PMID: 37288573
- PMCID: PMC10524979
- DOI: 10.1161/ATVBAHA.123.319145
Redox Dysregulation of Vascular Smooth Muscle Sirtuin-1 in Thoracic Aortic Aneurysm in Marfan Syndrome
Abstract
Background: Thoracic aortic aneurysms (TAAs) are abnormal aortic dilatations and a major cardiovascular complication of Marfan syndrome. We previously demonstrated a critical role for vascular smooth muscle (VSM) SirT1 (sirtuin-1), a lysine deacetylase, against maladaptive aortic remodeling associated with chronic oxidative stress and aberrant activation of MMPs (matrix metalloproteinases).
Methods: In this study, we investigated whether redox dysregulation of SirT1 contributed to the pathogenesis of TAA using fibrillin-1 hypomorphic mice (Fbn1mgR/mgR), an established model of Marfan syndrome prone to aortic dissection/rupture.
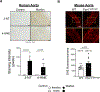
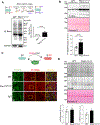
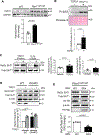
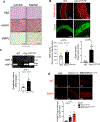
Results: Oxidative stress markers 3-nitrotyrosine and 4-hydroxynonenal were significantly elevated in aortas of patients with Marfan syndrome. Moreover, reversible oxidative post-translational modifications (rOPTM) of protein cysteines, particularly S-glutathionylation, were dramatically increased in aortas of Fbn1mgR/mgR mice, before induction of severe oxidative stress markers. Fbn1mgR/mgR aortas and VSM cells exhibited an increase in rOPTM of SirT1, coinciding with the upregulation of acetylated proteins, an index of decreased SirT1 activity, and increased MMP2/9 activity. Mechanistically, we demonstrated that TGFβ (transforming growth factor beta), which was increased in Fbn1mgR/mgR aortas, stimulated rOPTM of SirT1, decreasing its deacetylase activity in VSM cells. VSM cell-specific deletion of SirT1 in Fbn1mgR/mgR mice (SMKO-Fbn1mgR/mgR) caused a dramatic increase in aortic MMP2 expression and worsened TAA progression, leading to aortic rupture in 50% of SMKO-Fbn1mgR/mgR mice, compared with 25% of Fbn1mgR/mgR mice. rOPTM of SirT1, rOPTM-mediated inhibition of SirT1 activity, and increased MMP2/9 activity were all exacerbated by the deletion of Glrx (glutaredoxin-1), a specific deglutathionylation enzyme, while being corrected by overexpression of Glrx or of an oxidation-resistant SirT1 mutant in VSM cells.
Conclusions: Our novel findings strongly suggest a causal role of S-glutathionylation of SirT1 in the pathogenesis of TAA. Prevention or reversal of SirT1 rOPTM may be a novel therapeutic strategy to prevent TAA and TAA dissection/ruptures in individuals with Marfan syndrome, for which, thus far, no targeted therapy has been developed.
Keywords: Marfan syndrome; aorta; aortic aneurysm; lysine deacetylase; oxidative stress.
Conflict of interest statement
Figures



References
-
- Virani SS, Alonso A, Aparicio HJ, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. - PubMed
-
- Zúñiga-Muñoz AM, Pérez-Torres I, Guarner-Lans V, Núñez-Garrido E, Velázquez Espejel R, Huesca-Gómez C, Gamboa-Ávila R, Soto ME. Glutathione system participation in thoracic aneurysms from patients with Marfan syndrome. Vasa. 2017;46:177–186. - PubMed
-
- Jiménez-Altayó F, Meirelles T, Crosas-Molist E, et al. Redox stress in Marfan syndrome: Dissecting the role of the NADPH oxidase NOX4 in aortic aneurysm. Free Radic Biol Med. 2018;118:44–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

