Neoadjuvant immunochemotherapy for locally advanced resectable oral squamous cell carcinoma: a prospective single-arm trial (Illuminate Trial)
- PMID: 37288582
- PMCID: PMC10442116
- DOI: 10.1097/JS9.0000000000000489
Neoadjuvant immunochemotherapy for locally advanced resectable oral squamous cell carcinoma: a prospective single-arm trial (Illuminate Trial)
Abstract
Background: Locally advanced oral squamous cell carcinoma (LAOSCC) is associated with a high rate of recurrence and poor survival. Given the recent successes of neoadjuvant immunochemotherapy (NAICT) in solid tumors, it is promising to use this treatment modality to achieve a better pathological response and improve the survival of LAOSCC, and clinical evidence is needed to assess its safety and efficacy.
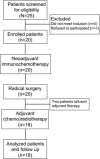
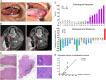
Patients and methods: A prospective trial of NAICT with toripalimab (PD-1 inhibitor) and albumin paclitaxel/cisplatin (TTP) was conducted in patients with clinical stage III and IVA OSCC. Intravenous albumin paclitaxel (260 mg/m 2 ), cisplatin (75 mg/m 2 ), and toripalimab (240 mg) were given in sequence on day 1 of each 21 day cycle for two cycles, followed by radical surgery and risk-adapted adjuvant (chemo)radiotherapy. The primary endpoints were safety and major pathological response (MPR). Targeted next generation sequencing and multiplex immunofluorescence were performed to assess clinical molecular characteristics and the tumor immune microenvironment in the pre-NAICT and post-NAICT tumor samples.
Results: Twenty patients were enrolled. NAICT was well-tolerated with a low incidence of grades 3-4 adverse events in three patients. The completion rates of NAICT and subsequent R0 resection were 100%. The MPR rate was 60%, including a 30% pathological complete response. MPR was achieved in all four patients with a combined positive score of PD-L1>10. The density of tertiary lymphatic structure in post-NAICT tumor samples predicted the pathological response to NAICT. During the median 23-month follow-up, the disease-free survival was 90%, and the overall survival was 95%.
Conclusions: NAICT with the TTP protocol in LAOSCC is feasible and well tolerated, with a promising MPR and no obstruction on subsequent surgery. This trial is supportive of further randomized trials using NAICT in LAOSCC.
Trial registration: ClinicalTrials.gov NCT04473716.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures

References
-
- Chow LQM. Head and neck cancer. N Engl J Med 2020;382:60–72. - PubMed
-
- D’Cruz AK, Vaish R, Dhar H. Oral cancers: current status. Oral Oncol 2018;87:64–69. - PubMed
-
- Kelly ZR, Gorantla VC, Davar D. The role of neoadjuvant therapy in melanoma. Curr Oncol Rep 2020;22:80. - PubMed
-
- Harbeck N, Gnant M. Breast cancer. Lancet 2017;389:1134–1150. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

