Targeting myocardial equilibrative nucleoside transporter ENT1 provides cardioprotection by enhancing myeloid Adora2b signaling
- PMID: 37288658
- PMCID: PMC10393224
- DOI: 10.1172/jci.insight.166011
Targeting myocardial equilibrative nucleoside transporter ENT1 provides cardioprotection by enhancing myeloid Adora2b signaling
Abstract
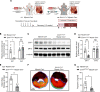
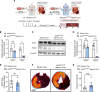
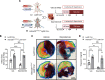
Previous studies implicate extracellular adenosine signaling in attenuating myocardial ischemia and reperfusion injury (IRI). This extracellular adenosine signaling is terminated by its uptake into cells by equilibrative nucleoside transporters (ENTs). Thus, we hypothesized that targeting ENTs would function to increase cardiac adenosine signaling and concomitant cardioprotection against IRI. Mice were exposed to myocardial ischemia and reperfusion injury. Myocardial injury was attenuated in mice treated with the nonspecific ENT inhibitor dipyridamole. A comparison of mice with global Ent1 or Ent2 deletion showed cardioprotection only in Ent1-/- mice. Moreover, studies with tissue-specific Ent deletion revealed that mice with myocyte-specific Ent1 deletion (Ent1loxP/loxP Myosin Cre+ mice) experienced smaller infarct sizes. Measurements of cardiac adenosine levels demonstrated that postischemic elevations of adenosine persisted during reperfusion after targeting ENTs. Finally, studies in mice with global or myeloid-specific deletion of the Adora2b adenosine receptor (Adora2bloxP/loxP LysM Cre+ mice) implied that Adora2b signaling on myeloid-inflammatory cells in cardioprotection provided by ENT inhibition. These studies reveal a previously unrecognized role for myocyte-specific ENT1 in cardioprotection by enhancing myeloid-dependent Adora2b signaling during reperfusion. Extension of these findings implicates adenosine transporter inhibitors in cardioprotection against ischemia and reperfusion injury.
Keywords: Cardiology; Cardiovascular disease; Hypoxia; Inflammation.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

