REST contributes to AKI-to-CKD transition through inducing ferroptosis in renal tubular epithelial cells
- PMID: 37288660
- PMCID: PMC10393228
- DOI: 10.1172/jci.insight.166001
REST contributes to AKI-to-CKD transition through inducing ferroptosis in renal tubular epithelial cells
Abstract
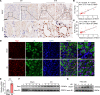
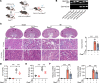
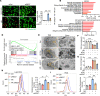
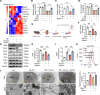
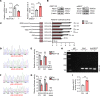
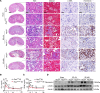
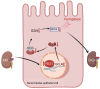
Ischemic-reperfusion injury (IRI) is a major pathogenic factor in acute kidney injury (AKI), which directly leads to the hypoxic injury of renal tubular epithelial cells (RTECs). Although emerging studies suggest repressor element 1-silencing transcription factor (REST) as a master regulator of gene repression under hypoxia, its role in AKI remains elusive. Here, we found that REST was upregulated in AKI patients, mice, and RTECs, which was positively associated with the degree of kidney injury, while renal tubule-specific knockout of Rest significantly alleviated AKI and its progression to chronic kidney disease (CKD). Subsequent mechanistic studies indicated that suppression of ferroptosis was responsible for REST-knockdown-induced amelioration of hypoxia-reoxygenation injury, during which process Cre-expressing adenovirus-mediated REST downregulation attenuated ferroptosis through upregulating glutamate-cysteine ligase modifier subunit (GCLM) in primary RTECs. Further, REST transcriptionally repressed GCLM expression via directly binding to its promoter region. In conclusion, our findings revealed the involvement of REST, a hypoxia regulatory factor, in AKI-to-CKD transition and identified the ferroptosis-inducing effect of REST, which may serve as a promising therapeutic target for ameliorating AKI and its progression to CKD.
Keywords: Fibrosis; Hypoxia; Nephrology.
Conflict of interest statement
Figures


References
-
- Gao L, et al. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther. 2020;5(1):9. doi: 10.1038/s41392-020-0106-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

