Emerging Chemical Biology of Protein Persulfidation
- PMID: 37288744
- PMCID: PMC10433728
- DOI: 10.1089/ars.2023.0352
Emerging Chemical Biology of Protein Persulfidation
Erratum in
-
Correction to: Emerging Chemical Biology of Protein Persulfidation by Vignane and Filipovic. Antioxid Redox Signal 2023;39(1-3):19-39. DOI: 10.1089/ars.2023.0352.Antioxid Redox Signal. 2023 Sep;39(7-9):620. doi: 10.1089/ars.2023.0352.correx. Epub 2023 Aug 18. Antioxid Redox Signal. 2023. PMID: 37594765 Free PMC article. No abstract available.
Abstract
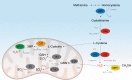
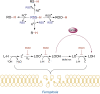
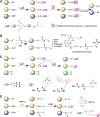
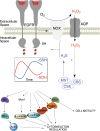
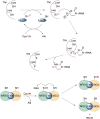
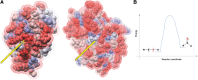
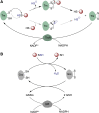
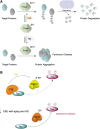
Significance: Protein persulfidation (the formation of RSSH), an evolutionarily conserved oxidative posttranslational modification in which thiol groups in cysteine residues are converted into persulfides, has emerged as one of the main mechanisms through which hydrogen sulfide (H2S) conveys its signaling. Recent Advances: New methodological advances in persulfide labeling started unraveling the chemical biology of this modification and its role in (patho)physiology. Some of the key metabolic enzymes are regulated by persulfidation. RSSH levels are important for the cellular defense against oxidative injury, and they decrease with aging, leaving proteins vulnerable to oxidative damage. Persulfidation is dysregulated in many diseases. Critical Issues: A relatively new field of signaling by protein persulfidation still has many unanswered questions: the mechanism(s) of persulfide formation and transpersulfidation and the identification of "protein persulfidases," the improvement of methods to monitor RSSH changes and identify protein targets, and understanding the mechanisms through which this modification controls important (patho)physiological functions. Future Directions: Deep mechanistic studies using more selective and sensitive RSSH labeling techniques will provide high-resolution structural, functional, quantitative, and spatiotemporal information on RSSH dynamics and help with better understanding how H2S-derived protein persulfidation affects protein structure and function in health and disease. This knowledge could pave the way for targeted drug design for a wide variety of pathologies. Antioxid. Redox Signal. 39, 19-39.
Keywords: aging; cysteine; hydrogen sulfide; posttranslational modifications; protein persulfidation; redox signaling.
Conflict of interest statement
No competing financial interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
