Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus
- PMID: 37288876
- PMCID: PMC10286687
- DOI: 10.1080/22221751.2023.2223669
Three neutralizing mAbs induced by MPXV A29L protein recognizing different epitopes act synergistically against orthopoxvirus
Abstract
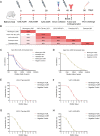
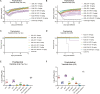
The worldwide outbreak of the monkeypox virus (MPXV) has become a "Public Health Emergency of International Concern" (PHEIC). Severe monkeypox virus infection can be fatal, however, effective therapeutic methods are yet to be developed. Mice were immunized with A35R protein and A29L protein of MPXV, and the binding and neutralizing activities of the immune sera against poxvirus-associated antigens and viruses were identified. A29L protein and A35R protein-specific monoclonal antibodies (mAbs) were generated and their antiviral activities of these mAbs were characterized in vitro and in vivo. Immunization with the MPXV A29L protein and A35R protein induced neutralizing antibodies against the orthopoxvirus in mice. None of the mAbs screened in this study against A35R could effectively neutralize the vaccinia virus (VACV), while three mAbs against A29L protein, 9F8, 3A1 and 2D1 were confirmed to have strong broad binding and neutralizing activities against orthopoxvirus, among which 9F8 showed the best neutralizing activity. 9F8, 3A1, and 2D1 recognized different epitopes on MPXV A29L protein, showing synergistic antiviral activity in vitro against the VACV Tian Tan and WR strains; the best activity was observed when the three antibodies were combined. In the vivo antiviral prophylactic and therapeutic experiments, 9F8 showed complete protective activity, whereas 3A1 and 2D1 showed partial protective activity. Similarly, the three antibodies showed synergistic antiviral protective activity against the two VACVs. In conclusion, three mAbs recognized different epitopes on MPXV A29L protein were developed and showed synergistic effects against orthopoxvirus.
Keywords: A29L protein; MPXV; Neutralizing mAbs; orthopoxvirus; synergistic effects.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Krupovic M, Cvirkaite-Krupovic V, Bamford DH.. Protein A33 responsible for antibody-resistant spread of vaccinia virus is homologous to C-type lectin-like proteins. Virus Res 2010;151(1):97–101. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
