A new tractable method for generating human alveolar macrophage-like cells in vitro to study lung inflammatory processes and diseases
- PMID: 37288969
- PMCID: PMC10470505
- DOI: 10.1128/mbio.00834-23
A new tractable method for generating human alveolar macrophage-like cells in vitro to study lung inflammatory processes and diseases
Abstract
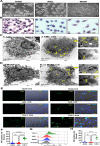
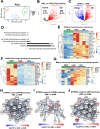
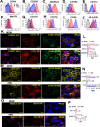
Alveolar macrophages (AMs) are unique lung resident cells that contact airborne pathogens and environmental particulates. The contribution of human AMs (HAMs) to pulmonary diseases remains poorly understood due to the difficulty in accessing them from human donors and their rapid phenotypic change during in vitro culture. Thus, there remains an unmet need for cost-effective methods for generating and/or differentiating primary cells into a HAM phenotype, particularly important for translational and clinical studies. We developed cell culture conditions that mimic the lung alveolar environment in humans using lung lipids, that is, Infasurf (calfactant, natural bovine surfactant) and lung-associated cytokines (granulocyte macrophage colony-stimulating factor, transforming growth factor-β, and interleukin 10) that facilitate the conversion of blood-obtained monocytes to an AM-like (AML) phenotype and function in tissue culture. Similar to HAM, AML cells are particularly susceptible to both Mycobacterium tuberculosis and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. This study reveals the importance of alveolar space components in the development and maintenance of HAM phenotype and function and provides a readily accessible model to study HAM in infectious and inflammatory disease processes, as well as therapies and vaccines. IMPORTANCE Millions die annually from respiratory disorders. Lower respiratory track gas-exchanging alveoli maintain a precarious balance between fighting invaders and minimizing tissue damage. Key players herein are resident AMs. However, there are no easily accessible in vitro models of HAMs, presenting a huge scientific challenge. Here, we present a novel model for generating AML cells based on differentiating blood monocytes in a defined lung component cocktail. This model is non-invasive, significantly less costly than performing a bronchoalveolar lavage, yields more AML cells than HAMs per donor, and retains their phenotype in culture. We have applied this model to early studies of M. tuberculosis and SARS-CoV-2. This model will significantly advance respiratory biology research.
Keywords: Mycobacterium tuberculosis; SARS-CoV-2; alveolar macrophage-like (AML) cells; human alveolar macrophages; lung cytokines; oxidative phosphorylation; surfactant.
Figures





Update of
-
A new tractable method for generating Human Alveolar Macrophage Like cells in vitro to study lung inflammatory processes and diseases.bioRxiv [Preprint]. 2023 Apr 6:2023.04.05.535806. doi: 10.1101/2023.04.05.535806. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0083423. doi: 10.1128/mbio.00834-23. PMID: 37066199 Free PMC article. Updated. Preprint.
References
-
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804. doi:10.1016/j.immuni.2013.04.004 - DOI - PMC - PubMed
-
- Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. 2013. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210:1977–1992. doi:10.1084/jem.20131199 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
