Neoadjuvant Doxorubicin-Paclitaxel Combined Chemotherapy in Patients with Inoperable Stage III Breast Cancer: A Retrospective Cohort Study with 10 Years of Follow-Up in Vietnam
- PMID: 37289321
- PMCID: PMC10447719
- DOI: 10.1007/s40487-023-00233-8
Neoadjuvant Doxorubicin-Paclitaxel Combined Chemotherapy in Patients with Inoperable Stage III Breast Cancer: A Retrospective Cohort Study with 10 Years of Follow-Up in Vietnam
Abstract
Introduction: The combination of doxorubicin and paclitaxel (AP) is widely used in our country for the neoadjuvant treatment of breast cancer as well as metastatic breast cancer. The AP regimen has shown promise as a neoadjuvant therapy for breast cancer that improves pathological complete response (pCR), increases the rate of conservative surgery, and improves the survival of patients. However, up to now, no research has evaluated the response of this regimen for the neoadjuvant treatment of advanced breast cancer, especially with a 10-year period of follow-up.
Methods: This retrospective analysis reviewed 126 patients with inoperable stage III breast cancer who received neoadjuvant chemotherapy with doxorubicin 50 mg/m2 plus paclitaxel 175 mg/m2 every 3 weeks for a maximum of six courses followed by surgery. pCR was evaluated. Survival was analyzed for all breast cancer patients using Kaplan-Meier and log-rank models.
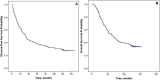
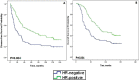
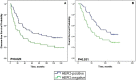
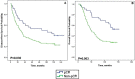
Results: Of 126 women treated with neoadjuvant chemotherapy (NAC), the overall pCR rate was 25.4% and was significantly higher in patients with tumor stage cT1-T2, hormone receptor-negative (HR-negative), and human epidermal growth factor receptor 2 (HER2)-positive disease. Patients achieving pCR had significantly longer disease-free survival (DFS) and overall survival (OS). Ten-year DFS rates were 43.8% vs. 25.0% (p = 0.030) and 10-year OS rates were 59.4% vs. 28.9% (p = 0.003) for patients with pCR and non-pCR, respectively. The cumulative 10-year DFS was 19.6% for patients with HR-negative disease and 37.3% for those with HR-positive disease. Achieving pCR was associated with improved 10-year OS and DFS. Several clinicopathological features were closely associated with pCR in the inoperable stage III breast cancer patients who were treated by neoadjuvant chemotherapy.
Conclusion: Achieving pCR was associated with improved 10-year OS and DFS. Patients with advanced breast cancer with HR-negative and HER2-positive status who benefited from the AP neoadjuvant therapy regimen were significantly more likely to achieve pCR.
Keywords: Advanced breast cancer; Doxorubicin-paclitaxel regimen; Neoadjuvant chemotherapy; Pathological complete response.
© 2023. The Author(s).
Conflict of interest statement
Duc Thanh Le, Lap Thanh Bui, Kien Hung Do, Tu Anh Do, Giang Le Tran and Chu Van Nguyen declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Mai DTT, Junda T, Sumdaengrit B. Symptom experiences and quality of life of Vietnamese patients with advanced cancer. J Health Sci Res. 2020;14(3):145–156.
-
- Le TD, Carney PA, Lee-Lin F, Mori M, Chen Z, Leung H, et al. Differences in knowledge, attitudes, beliefs, and perceived risks regarding colorectal cancer screening among Chinese, Korean, and Vietnamese sub-groups. J Community Health. 2014;39(2):248–265. doi: 10.1007/s10900-013-9776-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

