Advances in Osteoporosis Therapy: Focus on Osteoanabolic Agents, Secondary Fracture Prevention, and Perioperative Bone Health
- PMID: 37289382
- PMCID: PMC10393898
- DOI: 10.1007/s11914-023-00793-8
Advances in Osteoporosis Therapy: Focus on Osteoanabolic Agents, Secondary Fracture Prevention, and Perioperative Bone Health
Abstract
Purpose of review: This review summarizes recently published data and other developments around osteoanabolic osteoporosis therapies in patients with very high fracture risk, including those undergoing bone-related surgery.
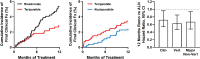
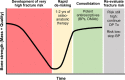
Recent findings: Two osteoanabolic agents, abaloparatide and romosozumab, were recently approved for treatment of patients with osteoporosis at high fracture risk. These agents, along with teriparatide, are valuable for primary and secondary fracture prevention. Orthopedic surgeons are well positioned to facilitate secondary fracture prevention via referrals to fracture liaison services or other bone health specialist colleagues. This review aims to help surgeons understand how to identify patients with sufficiently high fracture risk to warrant consideration of osteoanabolic therapy. Recent evidence around the perioperative use and potential benefits of osteoanabolic agents in fracture healing and other orthopedic settings (e.g., spinal fusion and arthroplasty) in individuals with osteoporosis is also discussed. Osteoanabolic agents should be considered for patients with osteoporosis at very high fracture risk, including those with prior osteoporotic fractures and those with poor bone health who are undergoing bone-related surgery.
Keywords: Abaloparatide; Orthopedic surgery; Romosozumab; Spine surgery; Teriparatide.
© 2023. The Author(s).
Conflict of interest statement
Dr. Kostenuik reports past and/or current consulting relationships and previous employment with Amgen and Radius Health, and consulting relationships with Mesentech Inc, AbbVie, Ascendis, Beren, UCB, Myovant, and Angitia Biopharmaceuticals. Dr. Kostenuik is also the CEO and co-founder of Ortheus Inc., with patents pending. Dr. Binkley reports research support to his institution from Radius Health and Amgen, and consulting for Amgen. Dr. Anderson reports personal fees from Amgen, Radius Medical, and Medtronic; grant support from Radius Medical; and stock in Titan Spine. Radius Health sponsored open access. This article received no other industry support or input and reflects the authors’ independent opinions and interpretations.
Figures
References
-
- Prevention and management of osteoporosis: report of a WHO scientific group: World Health Organization, 2003.
-
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26:1–46. doi: 10.4158/GL-2020-0524SUPPL. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

