Establishing severe acute respiratory infection (SARI) surveillance in a sentinel hospital, Ireland, 2021 to 2022
- PMID: 37289427
- PMCID: PMC10318943
- DOI: 10.2807/1560-7917.ES.2023.28.23.2200740
Establishing severe acute respiratory infection (SARI) surveillance in a sentinel hospital, Ireland, 2021 to 2022
Abstract
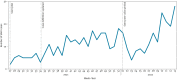
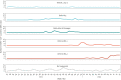
BackgroundIn 2020, due to the COVID-19 pandemic, the European Centre for Disease Prevention and Control (ECDC) accelerated development of European-level severe acute respiratory infection (SARI) surveillance.AimWe aimed to establish SARI surveillance in one Irish hospital as part of a European network E-SARI-NET.MethodsWe used routine emergency department records to identify cases in one adult acute hospital. The SARI case definition was adapted from the ECDC clinical criteria for a possible COVID-19 case. Clinical data were collected using an online questionnaire. Cases were tested for SARS-CoV-2, influenza and respiratory syncytial virus (RSV), including whole genome sequencing (WGS) on SARS-CoV-2 RNA-positive samples and viral characterisation/sequencing on influenza RNA-positive samples. Descriptive analysis was conducted for SARI cases hospitalised between July 2021 and April 2022.ResultsOverall, we identified 437 SARI cases, the incidence ranged from two to 28 cases per week (0.7-9.2/100,000 hospital catchment population). Of 431 cases tested for SARS-CoV-2 RNA, 226 (52%) were positive. Of 349 (80%) cases tested for influenza and RSV RNA, 15 (4.3%) were positive for influenza and eight (2.3%) for RSV. Using WGS, we identified Delta- and Omicron-dominant periods. The resource-intensive nature of manual clinical data collection, specimen management and laboratory supply shortages for influenza and RSV testing were challenging.ConclusionWe successfully established SARI surveillance as part of E-SARI-NET. Expansion to additional sentinel sites is planned following formal evaluation of the existing system. SARI surveillance requires multidisciplinary collaboration, automated data collection where possible, and dedicated personnel resources, including for specimen management.
Keywords: COVID-19; Influenza; Ireland; Severe Acute Respiratory Infection; Syndromic surveillance; respiratory syncytial virus, RSV.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO). International health regulations. Geneva: WHO; 2005. Available from: https://www.who.int/publications/i/item/9789241580410
-
- World Health Organization Regional Office for Europe (WHO/Europe). WHO Regional Office for Europe guidance for sentinel influenza surveillance in humans. Copenhagen: WHO/Europe; 2011. Available from: https://apps.who.int/iris/handle/10665/349780
-
- Henning KJ. What is syndromic surveillance? MMWR Suppl. 2004;53:5-11. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
