Patterns of antibiotic use, pathogens, and prediction of mortality in hospitalized neonates and young infants with sepsis: A global neonatal sepsis observational cohort study (NeoOBS)
- PMID: 37289666
- PMCID: PMC10249878
- DOI: 10.1371/journal.pmed.1004179
Patterns of antibiotic use, pathogens, and prediction of mortality in hospitalized neonates and young infants with sepsis: A global neonatal sepsis observational cohort study (NeoOBS)
Abstract
Background: There is limited data on antibiotic treatment in hospitalized neonates in low- and middle-income countries (LMICs). We aimed to describe patterns of antibiotic use, pathogens, and clinical outcomes, and to develop a severity score predicting mortality in neonatal sepsis to inform future clinical trial design.
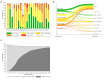
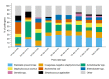
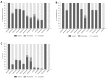
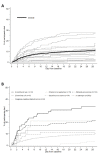
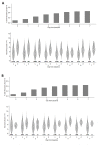
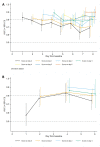
Methods and findings: Hospitalized infants <60 days with clinical sepsis were enrolled during 2018 to 2020 by 19 sites in 11 countries (mainly Asia and Africa). Prospective daily observational data was collected on clinical signs, supportive care, antibiotic treatment, microbiology, and 28-day mortality. Two prediction models were developed for (1) 28-day mortality from baseline variables (baseline NeoSep Severity Score); and (2) daily risk of death on IV antibiotics from daily updated assessments (NeoSep Recovery Score). Multivariable Cox regression models included a randomly selected 85% of infants, with 15% for validation. A total of 3,204 infants were enrolled, with median birth weight of 2,500 g (IQR 1,400 to 3,000) and postnatal age of 5 days (IQR 1 to 15). 206 different empiric antibiotic combinations were started in 3,141 infants, which were structured into 5 groups based on the World Health Organization (WHO) AWaRe classification. Approximately 25.9% (n = 814) of infants started WHO first line regimens (Group 1-Access) and 13.8% (n = 432) started WHO second-line cephalosporins (cefotaxime/ceftriaxone) (Group 2-"Low" Watch). The largest group (34.0%, n = 1,068) started a regimen providing partial extended-spectrum beta-lactamase (ESBL)/pseudomonal coverage (piperacillin-tazobactam, ceftazidime, or fluoroquinolone-based) (Group 3-"Medium" Watch), 18.0% (n = 566) started a carbapenem (Group 4-"High" Watch), and 1.8% (n = 57) a Reserve antibiotic (Group 5, largely colistin-based), and 728/2,880 (25.3%) of initial regimens in Groups 1 to 4 were escalated, mainly to carbapenems, usually for clinical deterioration (n = 480; 65.9%). A total of 564/3,195 infants (17.7%) were blood culture pathogen positive, of whom 62.9% (n = 355) had a gram-negative organism, predominantly Klebsiella pneumoniae (n = 132) or Acinetobacter spp. (n = 72). Both were commonly resistant to WHO-recommended regimens and to carbapenems in 43 (32.6%) and 50 (71.4%) of cases, respectively. MRSA accounted for 33 (61.1%) of 54 Staphylococcus aureus isolates. Overall, 350/3,204 infants died (11.3%; 95% CI 10.2% to 12.5%), 17.7% if blood cultures were positive for pathogens (95% CI 14.7% to 21.1%, n = 99/564). A baseline NeoSep Severity Score had a C-index of 0.76 (0.69 to 0.82) in the validation sample, with mortality of 1.6% (3/189; 95% CI: 0.5% to 4.6%), 11.0% (27/245; 7.7% to 15.6%), and 27.3% (12/44; 16.3% to 41.8%) in low (score 0 to 4), medium (5 to 8), and high (9 to 16) risk groups, respectively, with similar performance across subgroups. A related NeoSep Recovery Score had an area under the receiver operating curve for predicting death the next day between 0.8 and 0.9 over the first week. There was significant variation in outcomes between sites and external validation would strengthen score applicability.
Conclusion: Antibiotic regimens used in neonatal sepsis commonly diverge from WHO guidelines, and trials of novel empiric regimens are urgently needed in the context of increasing antimicrobial resistance (AMR). The baseline NeoSep Severity Score identifies high mortality risk criteria for trial entry, while the NeoSep Recovery Score can help guide decisions on regimen change. NeoOBS data informed the NeoSep1 antibiotic trial (ISRCTN48721236), which aims to identify novel first- and second-line empiric antibiotic regimens for neonatal sepsis.
Trial registration: ClinicalTrials.gov, (NCT03721302).
Copyright: © 2023 Russell et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Members of the funder (MB, NK, SE) participated as authors on the study, reviewed the manuscript, and approved the final manuscript as submitted. All other authors have declared that no competing interests exist.
Figures

References
-
- Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al.. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:731–741. doi: 10.1016/S1473-3099(14)70804-7 - DOI - PMC - PubMed
-
- Breiman RF, Blau DM, Mutevedzi P, Akelo V, Mandomando I, Ogbuanu IU, et al.. Postmortem investigations and identification of multiple causes of child deaths: An analysis of findings from the Child Health and Mortality Prevention Surveillance (CHAMPS) network. PLoS Med. 2021;18:e1003814. 10.1371/journal.pmed.1003814 - DOI - PMC - PubMed
-
- Gabrysch S, Nesbitt RC, Schoeps A, Hurt L, Soremekun S, Edmond K, et al.. Does facility birth reduce maternal and perinatal mortality in Brong Ahafo, Ghana? A secondary analysis using data on 119 244 pregnancies from two cluster-randomised controlled trials. Lancet Glob Health. 2019;7:e1074–e1087. doi: 10.1016/S2214-109X(19)30165-2 - DOI - PMC - PubMed
-
- Okomo U, Akpalu ENK, Le Doare K, Roca A, Cousens S, Jarde A, et al.. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019. [cited 2019 Sep 15]. doi: 10.1016/S1473-3099(19)30414-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

