Scalable cryopreservation of infectious Cryptosporidium hominis oocysts by vitrification
- PMID: 37289815
- PMCID: PMC10284403
- DOI: 10.1371/journal.ppat.1011425
Scalable cryopreservation of infectious Cryptosporidium hominis oocysts by vitrification
Abstract
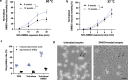
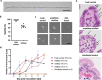
Cryptosporidium hominis is a serious cause of childhood diarrhea in developing countries. The development of therapeutics is impeded by major technical roadblocks including lack of cryopreservation and simple culturing methods. This impacts the availability of optimized/standardized singular sources of infectious parasite oocysts for research and human challenge studies. The human C. hominis TU502 isolate is currently propagated in gnotobiotic piglets in only one laboratory, which limits access to oocysts. Streamlined cryopreservation could enable creation of a biobank to serve as an oocyst source for research and distribution to other investigators requiring C. hominis. Here, we report cryopreservation of C. hominis TU502 oocysts by vitrification using specially designed specimen containers scaled to 100 μL volume. Thawed oocysts exhibit ~70% viability with robust excystation and 100% infection rate in gnotobiotic piglets. The availability of optimized/standardized sources of oocysts may streamline drug and vaccine evaluation by enabling wider access to biological specimens.
Copyright: © 2023 Jaskiewicz et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
We have read the journal’s policy and the authors of this manuscript have the following competing interests. Patent protection has been filed for the vitrification cassette technology.
Figures


References
-
- Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al.. The Global Enteric Multicenter Study (GEMS) of Diarrheal Disease in Infants and Young Children in Developing Countries: Epidemiologic and Clinical Methods of the Case/Control Study. Clinical Infectious Diseases. 2012;55:S232–S45. doi: 10.1093/cid/cis753 - DOI - PMC - PubMed
-
- Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, et al.. The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis. 2016;10(5):e0004729. - PMC - PubMed

