Source-tracking ESBL-producing bacteria at the maternity ward of Mulago hospital, Uganda
- PMID: 37289837
- PMCID: PMC10249850
- DOI: 10.1371/journal.pone.0286955
Source-tracking ESBL-producing bacteria at the maternity ward of Mulago hospital, Uganda
Abstract
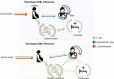
Introduction: Escherichia coli, Klebsiella pneumoniae and Enterobacter (EKE) are the leading cause of mortality and morbidity in neonates in Africa. The management of EKE infections remains challenging given the global emergence of carbapenem resistance in Gram-negative bacteria. This study aimed to investigate the source of EKE organisms for neonates in the maternity environment of a national referral hospital in Uganda, by examining the phenotypic and molecular characteristics of isolates from mothers, neonates, and maternity ward.
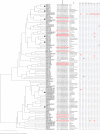
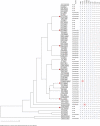
Methods: From August 2015 to August 2016, we conducted a cross-sectional study of pregnant women admitted for elective surgical delivery at Mulago hospital in Kampala, Uganda; we sampled (nose, armpit, groin) 137 pregnant women and their newborns (n = 137), as well as health workers (n = 67) and inanimate objects (n = 70 -beds, ventilator tubes, sinks, toilets, door-handles) in the maternity ward. Samples (swabs) were cultured for growth of EKE bacteria and isolates phenotypically/molecularly investigated for antibiotic sensitivity, as well as β-lactamase and carbapenemase activity. To infer relationships among the EKE isolates, spatial cluster analysis of phenotypic and genotypic susceptibility characteristics was done using the Ridom server.
Results: Gram-negative bacteria were isolated from 21 mothers (15%), 15 neonates (11%), 2 health workers (3%), and 13 inanimate objects (19%); a total of 131 Gram-negative isolates were identified of which 104 were EKE bacteria i.e., 23 (22%) E. coli, 50 (48%) K. pneumoniae, and 31 (30%) Enterobacter. Carbapenems were the most effective antibiotics as 89% (93/104) of the isolates were susceptible to meropenem; however, multidrug resistance was prevalent i.e., 61% (63/104). Furthermore, carbapenemase production and carbapenemase gene prevalence were low; 10% (10/104) and 6% (6/104), respectively. Extended spectrum β-lactamase (ESBL) production occurred in 37 (36%) isolates though 61 (59%) carried ESBL-encoding genes, mainly blaCTX-M (93%, 57/61) implying that blaCTX-M is the ideal gene for tracking ESBL-mediated resistance at Mulago. Additionally, spatial cluster analysis revealed isolates from mothers, new-borns, health workers, and environment with similar phenotypic/genotypic characteristics, suggesting transmission of multidrug-resistant EKE to new-borns.
Conclusion: Our study shows evidence of transmission of drug resistant EKE bacteria in the maternity ward of Mulago hospital, and the dynamics in the ward are more likely to be responsible for transmission but not individual mother characteristics. The high prevalence of drug resistance genes highlights the need for more effective infection prevention/control measures and antimicrobial stewardship programs to reduce spread of drug-resistant bacteria in the hospital, and improve patient outcomes.
Copyright: © 2023 Mayanja et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kayange N., Kamugisha E., Mwizamholya D. L., Jeremiah S., and Mshana S. E., “Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza- Tanzania,” BMC Pediatr., vol. 10, no. 1, p. 39, Jun. 2010, doi: 10.1186/1471-2431-10-39 - DOI - PMC - PubMed
-
- Tompkins K., Juliano J. J., and van Duin D., “Antimicrobial Resistance in Enterobacterales and Its Contribution to Sepsis in Sub-saharan Africa,” Front. Med., vol. 8, 2021, Accessed: Feb. 21, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fmed.2021.615649. doi: 10.3389/fmed.2021.615649 - DOI - DOI - PMC - PubMed
-
- Sheng W.-H., Badal R. E., Hsueh P.-R., and SMART Program, “Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: results of the study for Monitoring Antimicrobial Resistance Trends (SMART),” Antimicrob. Agents Chemother., vol. 57, no. 7, pp. 2981–2988, Jul. 2013, doi: 10.1128/AAC.00971-12 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

