Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial
- PMID: 37290035
- PMCID: PMC10419579
- DOI: 10.1200/JCO.23.00233
Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial
Abstract
Purpose: There is an unmet need for therapeutic options that prolong survival for patients with heavily pretreated, metastatic castration-resistant prostate cancer (mCRPC). The phase III, open-label KEYLYNK-010 study evaluated pembrolizumab plus olaparib versus a next-generation hormonal agent (NHA) for biomarker-unselected, previously treated mCRPC.
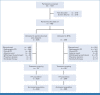
Methods: Eligible participants had mCRPC that progressed on or after abiraterone or enzalutamide (but not both) and docetaxel. Participants were randomly assigned (2:1) to pembrolizumab plus olaparib or NHA (abiraterone or enzalutamide). The dual primary end points were radiographic progression-free survival (rPFS) by blinded independent central review per Prostate Cancer Working Group-modified RECIST 1.1 and overall survival (OS). Time to first subsequent therapy (TFST) was a key secondary end point. Safety and objective response rate (ORR) were secondary end points.
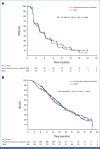
Results: Between May 30, 2019, and July 16, 2021, 529 participants were randomly assigned to pembrolizumab plus olaparib and 264 to NHA. At final rPFS analysis, median rPFS was 4.4 months (95% CI, 4.2 to 6.0) with pembrolizumab plus olaparib and 4.2 months (95% CI, 4.0 to 6.1) with NHA (hazard ratio [HR], 1.02 [95% CI, 0.82 to 1.25]; P = .55). At final OS analysis, median OS was 15.8 months (95% CI, 14.6 to 17.0) and 14.6 months (95% CI, 12.6 to 17.3), respectively (HR, 0.94 [95% CI, 0.77 to 1.14]; P = .26). At final TFST analysis, median TFST was 7.2 months (95% CI, 6.7 to 8.1) versus 5.7 months (95% CI, 5.0 to 7.1), respectively (HR, 0.86 [95% CI, 0.71 to 1.03]). ORR was higher with pembrolizumab plus olaparib versus NHA (16.8% v 5.9%). Grade ≥3 treatment-related adverse events occurred in 34.6% and 9.0% of participants, respectively.
Conclusion: Pembrolizumab plus olaparib did not significantly improve rPFS or OS versus NHA in participants with biomarker-unselected, heavily pretreated mCRPC. The study was stopped for futility. No new safety signals occurred.
Trial registration: ClinicalTrials.gov NCT03834519.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Prostate cancer (version 1.2023). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459
-
- Parker C, Castro E, Fizazi K, et al. : Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1119-1134, 2020 - PubMed
-
- George DJ, Sartor O, Miller K, et al. : Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer 18:284-294, 2020 - PubMed
-
- de Wit R, de Bono J, Sternberg CN, et al. : Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 381:2506-2518, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

