Targeting EB3-IP3R3 Interface with Cognate Peptide Protects from Acute Respiratory Distress Syndrome
- PMID: 37290041
- PMCID: PMC10557916
- DOI: 10.1165/rcmb.2022-0217OC
Targeting EB3-IP3R3 Interface with Cognate Peptide Protects from Acute Respiratory Distress Syndrome
Abstract
Acute respiratory distress syndrome (ARDS) is a lung disease characterized by acute onset of noncardiogenic pulmonary edema, hypoxemia, and respiratory insufficiency. The current treatment for ARDS is mainly supportive in nature, providing a critical need for targeted pharmacological management. We addressed this medical problem by developing a pharmacological treatment for pulmonary vascular leakage, a culprit of alveolar damage and lung inflammation. Our novel therapeutic target is the microtubule accessory factor EB3 (end binding protein 3), which contributes to pulmonary vascular leakage by amplifying pathological calcium signaling in endothelial cells in response to inflammatory stimuli. EB3 interacts with IP3R3 (inositol 1,4,5-trisphosphate receptor 3) and orchestrates calcium release from endoplasmic reticulum stores. Here, we designed and tested the therapeutic benefits of a 14-aa peptide named CIPRI (cognate IP3 receptor inhibitor), which disrupted EB3-IP3R3 interaction in vitro and in lungs of mice challenged with endotoxin. Treatment with CIPRI or depletion of IP3R3 in lung microvascular endothelial monolayers mitigated calcium release from endoplasmic reticulum stores and prevented a disassembly of vascular endothelial cadherin junctions in response to the proinflammatory mediator α-thrombin. Furthermore, intravenous administration of CIPRI in mice mitigated inflammation-induced lung injury, blocked pulmonary microvascular leakage, prevented activation of NFAT (nuclear factor of activated T cells) signaling, and reduced production of proinflammatory cytokines in the lung tissue. CIPRI also improved survival of mice from endotoxemia and polymicrobial sepsis. Together, these data demonstrate that targeting EB3-IP3R3 interaction with a cognate peptide is a promising strategy to address hyperpermeability of microvessels in inflammatory lung diseases.
Keywords: calcium signaling; endotoxemia; lung inflammation; sepsis; vascular leakage.
Figures

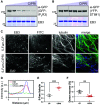
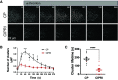
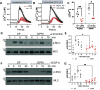

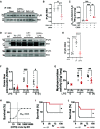
Comment in
-
Against the Waves: Blocking the EB3-IP3 Interaction Inhibits Calcium Waves in the Lung Endothelium.Am J Respir Cell Mol Biol. 2023 Oct;69(4):371-372. doi: 10.1165/rcmb.2023-0197ED. Am J Respir Cell Mol Biol. 2023. PMID: 37348847 Free PMC article. No abstract available.
References
-
- Fishman AP. Shock lung: a distinctive nonentity. Circulation . 1973;47:921–923. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med . 1994;149:818–824. - PubMed
-
- Brigham KL, Bowers R, Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res . 1979;45:292–297. - PubMed
-
- Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med . 1994;150:113–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

