Polymeric nanoparticle gel for intracellular mRNA delivery and immunological reprogramming of tumors
- PMID: 37290232
- PMCID: PMC10330908
- DOI: 10.1016/j.biomaterials.2023.122185
Polymeric nanoparticle gel for intracellular mRNA delivery and immunological reprogramming of tumors
Abstract
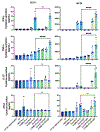
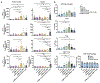
Immuno-oncology therapies have been of great interest with the goal of inducing sustained tumor regression, but clinical results have demonstrated the need for improved and widely applicable methods. An antigen-free method of cancer immunotherapy can stimulate the immune system to recruit lymphocytes and produce immunostimulatory factors without prior knowledge of neoantigens, while local delivery reduces the risk of systemic toxicity. To improve the interactions between tumor cells and cytotoxic lymphocytes, a gene delivery nanoparticle platform was engineered to reprogram the tumor microenvironment (TME) in situ to be more immunostimulatory by inducing tumor-associated antigen-presenting cells (tAPCs) to activate cytotoxic lymphocytes against the tumor. Biodegradable, lipophilic poly (beta-amino ester) (PBAE) nanoparticles were synthesized and used to co-deliver mRNA constructs encoding a signal 2 co-stimulatory molecule (4-1BBL) and a signal 3 immuno-stimulatory cytokine (IL-12), along with a nucleic acid-based immunomodulatory adjuvant. Nanoparticles are combined with a thermoresponsive block copolymer for gelation at the injection site for local NP retention at the tumor. The reprogramming nanoparticle gel synergizes with immune checkpoint blockade (ICB) to induce tumor regression and clearance in addition to resistance to tumor rechallenge at a distant site. In vitro and in vivo studies reveal increases in immunostimulatory cytokine production and recruitment of immune cells as a result of the nanoparticles. Intratumoral injection of nanoparticles encapsulating mRNA encoding immunostimulatory agents and adjuvants via an injectable thermoresponsive gel has great translational potential as an immuno-oncology therapy that can be accessible to a wide range of patients.
Keywords: Immuno-oncology; Immunoengineering; Immunotherapy; Nanoparticles; Nonviral gene delivery; mRNA.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Stephany Y. Tzeng reports a relationship with OncoSwitch Therapeutics, LLC that includes: equity or stocks. Jordan J. Green reports a relationship with OncoSwitch Therapeutics, LLC that includes: equity or stocks. Sarah Y. Neshat, Stephany Y. Tzeng, Jordan J. Green has patent pending to Johns Hopkins University.
Figures






References
-
- Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T, Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2021 191 19, 37–50 (2021). - PubMed
-
- Taefehshokr S, et al. , Cancer immunotherapy: Challenges and limitations. Pathol. - Res. Pract 229, 153723 (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

