A systematic review and meta-analysis of randomized controlled trials to evaluate plant-based omega-3 polyunsaturated fatty acids in nonalcoholic fatty liver disease patient biomarkers and parameters
- PMID: 37290426
- PMCID: PMC10777680
- DOI: 10.1093/nutrit/nuad054
A systematic review and meta-analysis of randomized controlled trials to evaluate plant-based omega-3 polyunsaturated fatty acids in nonalcoholic fatty liver disease patient biomarkers and parameters
Abstract
Context: Nonalcoholic fatty liver disease (NAFLD) is prevalent in 25-30% of British and European populations, representing a potential global public health crisis. Marine omega-3 (n-3) polyunsaturated fatty acids offer well-evidenced benefits to NAFLD biomarkers; however, the effect of plant-based n-3 has not been evaluated with a systematic review and meta-analysis.
Objective: The review aimed to systematically evaluate the effect of plant-based n-3 supplementation on NAFLD surrogate biomarkers and parameters.
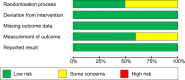
Data sources: Medline (EBSCO), PubMed, CINAHL (EBSCO), Cochrane Central Register of Controlled Trials, the International Clinical Trials Registry Platform, and Google Scholar databases were searched to identify randomized controlled trials published between January 1970 and March 2022 evaluating the impact of plant-based n-3 interventions on diagnosed NAFLD. The review followed the PRISMA checklist and is PROSPERO registered (CRD42021251980).
Data extraction: A random-effects model and generic inverse variance methods synthesized quantitative data, followed by a leave-one-out method for sensitivity analysis. We identified 986 articles; after the application of selection criteria, six studies remained with 362 patients with NAFLD.
Results: The meta-analysis showed that plant-based n-3 fatty acid supplementation significantly reduced alanine aminotransferase (ALT) (mean difference: 8.04 IU/L; 95% confidence interval: 14.70, 1.38; I2 = 48.61%) and plasma/serum triglycerides (44.51 mg/dL; 95% confidence interval: -76.93, -12.08; I2 = 69.93%), alongside body-composition markers in patients with NAFLD (P < 0.05).
Conclusion: Plant-based n-3 fatty acid supplementation improves ALT enzyme biomarkers, triglycerides, body mass index, waist circumference, and weight loss when combined with lifestyle interventions to increase physical activity and a calorie-controlled diet. Further research is needed to identify the most effective plant-based n-3 sources in larger numbers of patients with NAFLD over longer study durations.
Systematic review registration: PROSPERO registration no. CRD42021251980.
Keywords: nonalcoholic fatty liver disease; omega-3; plant-based; supplementation.
© The Author(s) 2023. Published by Oxford University Press on behalf of the International Life Sciences Institute.
Figures
















Similar articles
-
Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials.Medicine (Baltimore). 2018 Sep;97(37):e12271. doi: 10.1097/MD.0000000000012271. Medicine (Baltimore). 2018. PMID: 30212963 Free PMC article.
-
Omega-3 polyunsaturated fatty acids and nonalcoholic fatty liver disease in adults: A meta-analysis of randomized controlled trials.Clin Nutr. 2025 Jul;50:164-174. doi: 10.1016/j.clnu.2025.05.013. Epub 2025 May 20. Clin Nutr. 2025. PMID: 40441053
-
Curcumin supplementation effect on liver enzymes in patients with nonalcoholic fatty liver disease: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials.Nutr Rev. 2025 Jan 1;83(1):1-12. doi: 10.1093/nutrit/nuad166. Nutr Rev. 2025. PMID: 38213188
-
Efficacy and safety of omega-3 fatty acids on liver-related outcomes in patients with nonalcoholic fatty liver disease: A protocol for a systematic review and meta-analysis.Medicine (Baltimore). 2020 Jun 12;99(24):e20624. doi: 10.1097/MD.0000000000020624. Medicine (Baltimore). 2020. PMID: 32541499 Free PMC article.
-
Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials.PLoS One. 2016 Oct 6;11(10):e0162368. doi: 10.1371/journal.pone.0162368. eCollection 2016. PLoS One. 2016. PMID: 27711128 Free PMC article.
Cited by
-
Dynamics of Fatty Acid Composition in Lipids and Their Distinct Roles in Cardiometabolic Health.Biomolecules. 2025 May 10;15(5):696. doi: 10.3390/biom15050696. Biomolecules. 2025. PMID: 40427589 Free PMC article. Review.
-
Omega-3 Fatty Acids, Furan Fatty Acids, and Hydroxy Fatty Acid Esters: Dietary Bioactive Lipids with Potential Benefits for MAFLD and Liver Health.Nutrients. 2025 Mar 15;17(6):1031. doi: 10.3390/nu17061031. Nutrients. 2025. PMID: 40292496 Free PMC article. Review.
-
Role of amino acids in the regulation of hepatic gluconeogenesis and lipogenesis in metabolic dysfunctionassociated steatotic liver disease.Clin Mol Hepatol. 2025 Jul;31(3):771-795. doi: 10.3350/cmh.2025.0048. Epub 2025 Apr 16. Clin Mol Hepatol. 2025. PMID: 40239968 Free PMC article. Review.
-
Potential Therapeutic Strategies in the Treatment of Metabolic-Associated Fatty Liver Disease.Medicina (Kaunas). 2023 Oct 8;59(10):1789. doi: 10.3390/medicina59101789. Medicina (Kaunas). 2023. PMID: 37893507 Free PMC article. Review.
-
Profiling of RNA N6-Methyladenosine methylation reveals the critical role of m6A in betaine alleviating hepatic steatosis.Sci Rep. 2025 Mar 1;15(1):7298. doi: 10.1038/s41598-025-91573-0. Sci Rep. 2025. PMID: 40025137 Free PMC article.
References
-
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

