Platinum-combination chemotherapy with or without immune-checkpoint inhibitor in patients with postoperative recurrent non-small cell lung cancer previously treated with adjuvant platinum-doublet chemotherapy: A multicenter retrospective study
- PMID: 37290434
- PMCID: PMC10363783
- DOI: 10.1111/1759-7714.14992
Platinum-combination chemotherapy with or without immune-checkpoint inhibitor in patients with postoperative recurrent non-small cell lung cancer previously treated with adjuvant platinum-doublet chemotherapy: A multicenter retrospective study
Abstract
Background: Rechallenge with platinum-combination chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) after disease progression on platinum-combination chemotherapy occasionally leads to a favorable response. The efficacy and safety of platinum-combination chemotherapy with or without immune-checkpoint inhibitor (ICI) for patients with recurrent NSCLC after surgery followed by adjuvant platinum-doublet chemotherapy remains uncertain.
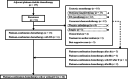
Methods: Patients who relapsed after surgery plus adjuvant platinum-doublet chemotherapy and received platinum-combination chemotherapy with or without ICI between April 2011 and March 2021 at four Nippon Medical School hospitals were retrospectively analyzed.
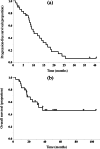
Results: Among 177 patients who received adjuvant platinum-doublet chemotherapy after surgery, a total of 30 patients who received platinum-combination rechemotherapy with or without ICI after relapse were included in this study. Seven patients received ICI-combined chemotherapy. The median disease-free survival (DFS) after surgery was 13.6 months. The objective response rate and disease-control rate were 46.7% and 80.0%, respectively. The median progression-free survival and overall survival were 10.2 and 37.5 months, respectively. Patients with longer DFS (≥12 months) had a better prognosis than others. The most common grade ≥3 toxicity associated with this treatment was neutropenia (33%). Grade ≥3 immune-related adverse events were pneumonitis (14%) and colitis (14%). Treatment-related deaths did not occur in this study.
Conclusion: Platinum-combination chemotherapy with or without ICI for patients with postoperative recurrent NSCLC who previously received adjuvant platinum-doublet chemotherapy was effective and safe. In particular, this therapy may be promising for patients with longer DFS.
Keywords: immune-checkpoint inhibitor; non-small cell lung cancer; platinum-combination chemotherapy; postoperative recurrence.
© 2023 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
M. Seike received funding from Taiho Pharmaceutical, Chugai Pharmaceutical, and MSD; and honoraria for lectures from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol‐Meyers Squibb, and MSD. T. Hirose received funding from Taiho Pharmaceutical, and Chugai Pharmaceutical; and honoraria for lectures from Taiho Pharmaceutical, Chugai Pharmaceutical, Bristol‐Meyers Squibb, and MSD. The other authors have no potential conflicts of interest.
Figures

Similar articles
-
A Phase 2 Study of Docetaxel, Ramucirumab, and Pembrolizumab for Patients With Metastatic or Recurrent Non-Small-Cell Lung Cancer (NSCLC) who Progressed on Platinum-Doublet and PD-1/PD-L1 Blockade.Clin Lung Cancer. 2022 Nov;23(7):e400-e404. doi: 10.1016/j.cllc.2022.06.003. Epub 2022 Jun 22. Clin Lung Cancer. 2022. PMID: 35863963 Clinical Trial.
-
Immune Checkpoint Inhibitor Therapy for Patients With Non-small Cell Lung Cancer Ineligible for Platinum Doublet Chemotherapy.Anticancer Res. 2024 Mar;44(3):1241-1245. doi: 10.21873/anticanres.16920. Anticancer Res. 2024. PMID: 38423671
-
Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: Case series and literature review.Thorac Cancer. 2020 Jul;11(7):1927-1933. doi: 10.1111/1759-7714.13483. Epub 2020 May 18. Thorac Cancer. 2020. PMID: 32421224 Free PMC article.
-
Immunotherapy Alone or in Combination with Chemotherapy as First-Line Treatment of Non-Small Cell Lung Cancer.Curr Treat Options Oncol. 2020 Jul 27;21(8):69. doi: 10.1007/s11864-020-00768-2. Curr Treat Options Oncol. 2020. PMID: 32720019 Review.
-
Platinum-doublet chemotherapy as second-line treatment for relapsed patients with small-cell lung cancer: A systematic review and meta-analysis.Lung Cancer. 2021 Jun;156:59-67. doi: 10.1016/j.lungcan.2021.04.013. Epub 2021 Apr 19. Lung Cancer. 2021. PMID: 33894495
References
-
- Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823–33. - PubMed
-
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. - PubMed
-
- Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. - PubMed
-
- Paz‐Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2040–51. - PubMed
-
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

