Elevated cfDNA after exercise is derived primarily from mature polymorphonuclear neutrophils, with a minor contribution of cardiomyocytes
- PMID: 37290439
- PMCID: PMC10313937
- DOI: 10.1016/j.xcrm.2023.101074
Elevated cfDNA after exercise is derived primarily from mature polymorphonuclear neutrophils, with a minor contribution of cardiomyocytes
Abstract
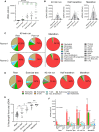
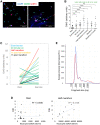
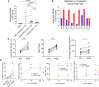
Strenuous physical exercise causes a massive elevation in the concentration of circulating cell-free DNA (cfDNA), which correlates with effort intensity and duration. The cellular sources and physiological drivers of this phenomenon are unknown. Using methylation patterns of cfDNA and associated histones, we show that cfDNA in exercise originates mostly in extramedullary polymorphonuclear neutrophils. Strikingly, cardiomyocyte cfDNA concentration increases after a marathon, consistent with elevated troponin levels and indicating low-level, delayed cardiac cell death. Physical impact, low oxygen levels, and elevated core body temperature contribute to neutrophil cfDNA release, while muscle contraction, increased heart rate, β-adrenergic signaling, or steroid treatment fail to cause elevation of cfDNA. Physical training reduces neutrophil cfDNA release after a standard exercise, revealing an inverse relationship between exercise-induced cfDNA release and training level. We speculate that the release of cfDNA from neutrophils in exercise relates to the activation of neutrophils in the context of exercise-induced muscle damage.
Keywords: ChIP-seq; chromatin; circulating cell-free DNA; exercise biology; fitness; inflammation; methylation; neutrophil extracellular traps; neutrophils; polymorphonuclear cells.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R. Sadeh, G.F., I.S., and N.F. are founders and/or employees of Senseera Inc. J.M., B.G., R. Shemer, and Y.D. have filed patents on cfDNA methylation analysis.
Figures





References
-
- Fischer C.P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 2006;12:6–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

