Theranostics in Hematooncology
- PMID: 37290799
- PMCID: PMC10315699
- DOI: 10.2967/jnumed.122.265199
Theranostics in Hematooncology
Abstract
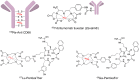
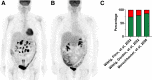
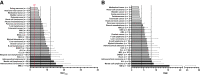
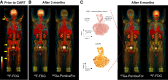
In the early 2000s, major clinical trials provided evidence of a favorable outcome from antibody-mediated radioimmunotherapy for hematologic neoplasms, which then led to Food and Drug Administration approval. For instance, the theranostic armamentarium for the referring hematooncologist now includes 90Y-ibritumomab tiuxetan for refractory low-grade follicular lymphoma or transformed B-cell non-Hodgkin lymphoma, as well as 131I-tositumomab for rituximab-refractory follicular lymphoma. Moreover, the first interim results of the SIERRA phase III trial reported beneficial effects from the use of 131I-anti-CD45 antibodies (Iomab-B) in refractory or relapsed acute myeloid leukemia. During the last decade, the concept of theranostics in hematooncology has been further expanded by C-X-C motif chemokine receptor 4-directed molecular imaging. Beyond improved detection rates of putative sites of disease, C-X-C motif chemokine receptor 4-directed PET/CT also selects candidates for radioligand therapy using β-emitting radioisotopes targeting the identical chemokine receptor on the lymphoma cell surface. Such image-piloted therapeutic strategies provided robust antilymphoma efficacy, along with desired eradication of the bone marrow niche, such as in patients with T- or B-cell lymphoma. As an integral part of the treatment plan, such radioligand therapy-mediated myeloablation also allows one to line up patients for stem cell transplantation, which leads to successful engraftment during the further treatment course. In this continuing education article, we provide an overview of the current advent of theranostics in hematooncology and highlight emerging clinical applications.
Keywords: C-X-C motif chemokine receptor 4; CXCR4; hematooncology; lymphoma; radioimmunotherapy; theranostics.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures
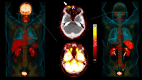
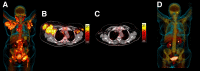
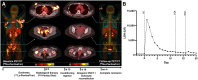
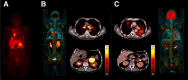
References
-
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. - PubMed
-
- Strosberg JR, Caplin ME, Kunz PL, et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:1752–1763. - PubMed
-
- Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
