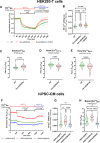
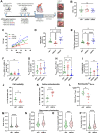
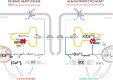
Fig. 4. S663A phosphorylation modulates Ca2+ signaling and function in mouse cardiomyocytes.
A Typical curves of [Ca2+]CYTO (cytosol) F/F0 ratio signals over time measured with Fluo-4, in rWT (green curve), rS663A (red curve) and rS663E (blue curve) rescued cardiomyocytes, in 2 mM Ca2+ electro-stimulation buffer for 30 s, followed by 20 s electro-stimulation at 1.0 Hz and 40 V, and prior to 5 mM caffeine stimulation at time 60 s. B Violin plots of the transient amplitude during electro-stimulation in rWT (N = 184 cells on 5 experimental days), rS663A (N = 253 cells on 5 experimental days) and rS663E (N = 260 cells on 6 experimental days). Median with interquartile range is shown and one-way ANOVA followed by Tukey’s multiple comparisons test was used to assess significance (ns p = 0.0666, **p = 0.0014, ****p < 0.0001). C Violin plots of the [Ca2+] maximal increase in rWT (N = 214 on 5 experimental days), rS663A (N = 164 cells on 5 experimental days) and rS663E (N = 257 cells on 6 experimental days). Median with interquartile range is shown and one-way ANOVA followed by Tukey’s multiple comparisons test was used to assess significance (ns p = 0.9759, *p = 0.0241, **p = 0.0063). D Violin plots of the rate of exponential decay after caffeine stimulation in rWT (N = 94 cells on 5 experimental days), rS663A (N = 118 cells on 5 experimental days) and rS663E (N = 145 cells on 6 experimental days). Median with interquartile range is shown and one-way ANOVA followed by Tukey’s multiple comparisons test was used to assess significance (ns p = 0.0666, **p = 0.0014, ****p < 0.0001). E Violin plot of basal [Ca2+]CYTO (cytosol) ratio signals measured with the ratiometric chemical Ca2+ indicator Fura-2, in rWT (N = 111 cells on 3 independent days), S663A (N = 66 cells on 3 independent days) and rS663E (N = 69 cells on 3 independent days). Median with interquartile range is shown and one-way ANOVA followed by Tukey’s multiple comparisons test was used to assess significance (ns p = 9012, ****p < 0.0001). F Current–voltage relationships of peak ICa,L normalized to membrane capacitance from rWT (green curve; N = 16), rS663A (red curve; N = 15), rS663E (blue curve; N = 15) and non-transgenic control (Ctr; gray curve; N = 17) cells isolated from 3 mice in each group. Data are expressed as mean ± CI 95%. Inserted on the left, representative traces of L-type Ca2+ current in rWT, rS663A, rS663E and Ctr cardiomyocytes during depolarizing steps spaced 10 mV apart and varying between 0 and +40 mV (uppermost traces) from a holding potential of −80 mV. G Average traces (±CI 95%) of INCX measured as the Li+-sensitive slow tail inward current recorded every 10 s when polarizing the cell to −80 mV after it has been depolarized 20 ms to −50 mV (to inactivate the fast sodium current), and then 30 ms to +10 mV (to activate ICa,L), from rWT (N = 24), rS663A (N = 24), rS663E (N = 23) and Ctr (C57Bl6J mice N = 27) cells isolated from 6, 7, 5 and 6 mice, respectively. Box (with median and interquartile range) and whiskers (10-90%) showing INCX densities measured after 20 ms of repolarization to −80 mV have been added on every trace. (***p < 0.001 vs Ctr). H Panel G corresponding boxes (with median and interquartile range) and whiskers (10–90%) with dot plots showing INCX densities measured as the integrated form of inward currents (135 ms integration starting 15 ms after the onset of repolarization). Expressed as mean ± CI 95%, membrane capacitances in pF were: rWT: 141.1 ± 11.9, N = 40; rS663A: 157.9 ± 14.7, N = 39; rS663E: 158.5 ± 15.3, N = 38, Ctr: 156.5 ± 13.6, N = 44. ***p < 0.001 indicates on G and H statistically significant differences compared to Ctr using the Kruskal–Wallis test followed by Dunn’s multiple comparisons test. I Representative records of inward tail current normalized to membrane capacitance recorded at −80 mV from rWT, rS663A, rS663E and Ctr cardiomyocytes in Na+ (P3) and Li+ (P5) external solution and after return in the Na+ external solution (P7, P9 and P11). J Time course of inward tail current densities measured 20 ms after the onset of the repolarization to −80 mV from rWT (N = 19), rS663A (N = 18), rS663E (N = 20) and Ctr (N = 21) cells isolated from same mice as in (F) and (G). First placed in external Na+ (P1–P3), the cells were then exposed to external Li+ (P4–P6), before being returned to external Na+ (from P7 to P16), Data are presented as median with interquartile range. The Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used, and only differences with Ctr were reported as significant. a, p < 0.01 at least for the 3 mutants versus Ctr, b, p < 0.05 at least for the 3 mutants versus Ctr, and c, p < 0.05 only for the rS663A mutant versus Ctr. K Panel J corresponding integrated NCX density currents from rWT, rS663A and rS663E mutant cardiomyocytes displayed in Na+ (P1–P3) and Li+ (P4–P6) external solution and after return in the Na+ external solution (from P7 to P16). Data are presented as box (median with interquartile range) and whiskers (10–90%). The Kruskal–Wallis test followed by Dunn’s multiple comparisons test was used and only differences with rS663A were reported as significant (P1: p = 0,0702, P2: p = 0,1466, P3: p = 0,0573, P4: p = 0,0964, P5: p = 0,4200, P6: p = 0,1806, P7: p = 0,0401, P8: p = 0,0113, P9: p = 0,0054, P10: p = 0,0007, P11: p = 0,0039, P12: p = 0,0033, P13: p = 0,0175, P14: p = 0,0318, P15: p = 0,2819 and P16: p = 0,1083, vs rS663A). Dash lines on F, G and I indicate zero current.