Fertilization controls tiller numbers via transcriptional regulation of a MAX1-like gene in rice cultivation
- PMID: 37291104
- PMCID: PMC10250342
- DOI: 10.1038/s41467-023-38670-8
Fertilization controls tiller numbers via transcriptional regulation of a MAX1-like gene in rice cultivation
Abstract
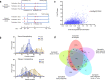
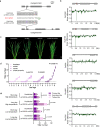
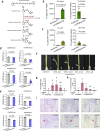
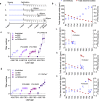
Fertilization controls various aspects of cereal growth such as tiller number, leaf size, and panicle size. However, despite such benefits, global chemical fertilizer use must be reduced to achieve sustainable agriculture. Here, based on field transcriptome data from leaf samples collected during rice cultivation, we identify fertilizer responsive genes and focus on Os1900, a gene orthologous to Arabidopsis thaliana MAX1, which is involved in strigolactone biosynthesis. Elaborate genetic and biochemical analyses using CRISPR/Cas9 mutants reveal that Os1900 together with another MAX1-like gene, Os5100, play a critical role in controlling the conversion of carlactone into carlactonoic acid during strigolactone biosynthesis and tillering in rice. Detailed analyses of a series of Os1900 promoter deletion mutations suggest that fertilization controls tiller number in rice through transcriptional regulation of Os1900, and that a few promoter mutations alone can increase tiller numbers and grain yields even under minor-fertilizer conditions, whereas a single defective os1900 mutation does not increase tillers under normal fertilizer condition. Such Os1900 promoter mutations have potential uses in breeding programs for sustainable rice production.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures