Uncovering the chiral bias of meteoritic isovaline through asymmetric photochemistry
- PMID: 37291172
- PMCID: PMC10250315
- DOI: 10.1038/s41467-023-39177-y
Uncovering the chiral bias of meteoritic isovaline through asymmetric photochemistry
Abstract
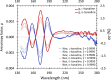
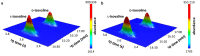
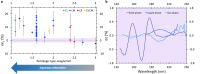
Systematic enrichments of L-amino acids in meteorites is a strong indication that biological homochirality originated beyond Earth. Although still unresolved, stellar UV circularly polarized light (CPL) is the leading hypothesis to have caused the symmetry breaking in space. This involves the differential absorption of left- and right-CPL, a phenomenon called circular dichroism, which enables chiral discrimination. Here we unveil coherent chiroptical spectra of thin films of isovaline enantiomers, the first step towards asymmetric photolysis experiments using a tunable laser set-up. As analogues to amino acids adsorbed on interstellar dust grains, CPL-helicity dependent enantiomeric excesses of up to 2% were generated in isotropic racemic films of isovaline. The low efficiency of chirality transfer from broadband CPL to isovaline could explain why its enantiomeric excess is not detected in the most pristine chondrites. Notwithstanding, small, yet consistent L-biases induced by stellar CPL would have been crucial for its amplification during aqueous alteration of meteorite parent bodies.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies.Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5487-92. doi: 10.1073/pnas.0811618106. Epub 2009 Mar 16. Proc Natl Acad Sci U S A. 2009. PMID: 19289826 Free PMC article.
-
Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues.Nature. 2002 Mar 28;416(6879):401-3. doi: 10.1038/416401a. Nature. 2002. PMID: 11919623
-
Chirality emergence in thin solid films of amino acids by polarized light from synchrotron radiation and free electron laser.Int J Mol Sci. 2009 Jul 7;10(7):3044-3064. doi: 10.3390/ijms10073044. Int J Mol Sci. 2009. PMID: 19742124 Free PMC article.
-
Photochirogenesis: photochemical models on the absolute asymmetric formation of amino acids in interstellar space.Phys Life Rev. 2011 Oct;8(3):307-30. doi: 10.1016/j.plrev.2011.08.005. Epub 2011 Sep 3. Phys Life Rev. 2011. PMID: 21924690 Review.
-
Chirality, photochemistry and the detection of amino acids in interstellar ice analogues and comets.Chem Soc Rev. 2012 Aug 21;41(16):5447-58. doi: 10.1039/c2cs35051c. Epub 2012 May 10. Chem Soc Rev. 2012. PMID: 22576562 Review.
Cited by
-
The astrochemical evolutionary traits of phospholipid membrane homochirality.Nat Rev Chem. 2024 Sep;8(9):652-664. doi: 10.1038/s41570-024-00627-w. Epub 2024 Jul 18. Nat Rev Chem. 2024. PMID: 39025922 Review.
-
The Mystery of Homochirality on Earth.Life (Basel). 2024 Mar 6;14(3):341. doi: 10.3390/life14030341. Life (Basel). 2024. PMID: 38541666 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

