Somatic mutations of CADM1 in aldosterone-producing adenomas and gap junction-dependent regulation of aldosterone production
- PMID: 37291193
- PMCID: PMC10260400
- DOI: 10.1038/s41588-023-01403-0
Somatic mutations of CADM1 in aldosterone-producing adenomas and gap junction-dependent regulation of aldosterone production
Abstract
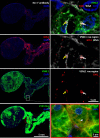
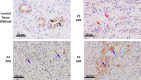
Aldosterone-producing adenomas (APAs) are the commonest curable cause of hypertension. Most have gain-of-function somatic mutations of ion channels or transporters. Herein we report the discovery, replication and phenotype of mutations in the neuronal cell adhesion gene CADM1. Independent whole exome sequencing of 40 and 81 APAs found intramembranous p.Val380Asp or p.Gly379Asp variants in two patients whose hypertension and periodic primary aldosteronism were cured by adrenalectomy. Replication identified two more APAs with each variant (total, n = 6). The most upregulated gene (10- to 25-fold) in human adrenocortical H295R cells transduced with the mutations (compared to wildtype) was CYP11B2 (aldosterone synthase), and biological rhythms were the most differentially expressed process. CADM1 knockdown or mutation inhibited gap junction (GJ)-permeable dye transfer. GJ blockade by Gap27 increased CYP11B2 similarly to CADM1 mutation. Human adrenal zona glomerulosa (ZG) expression of GJA1 (the main GJ protein) was patchy, and annular GJs (sequelae of GJ communication) were less prominent in CYP11B2-positive micronodules than adjacent ZG. Somatic mutations of CADM1 cause reversible hypertension and reveal a role for GJ communication in suppressing physiological aldosterone production.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
-
- Funder JW, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016;101:1889–1916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

