Hardwiring tissue-specific AAV transduction in mice through engineered receptor expression
- PMID: 37291262
- PMCID: PMC10333121
- DOI: 10.1038/s41592-023-01896-x
Hardwiring tissue-specific AAV transduction in mice through engineered receptor expression
Abstract
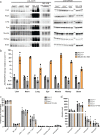
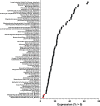
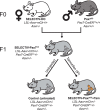
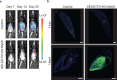
The development of transgenic mouse models that express genes of interest in specific cell types has transformed our understanding of basic biology and disease. However, generating these models is time- and resource-intensive. Here we describe a model system, SELective Expression and Controlled Transduction In Vivo (SELECTIV), that enables efficient and specific expression of transgenes by coupling adeno-associated virus (AAV) vectors with Cre-inducible overexpression of the multi-serotype AAV receptor, AAVR. We demonstrate that transgenic AAVR overexpression greatly increases the efficiency of transduction of many diverse cell types, including muscle stem cells, which are normally refractory to AAV transduction. Superior specificity is achieved by combining Cre-mediated AAVR overexpression with whole-body knockout of endogenous Aavr, which is demonstrated in heart cardiomyocytes, liver hepatocytes and cholinergic neurons. The enhanced efficacy and exquisite specificity of SELECTIV has broad utility in development of new mouse model systems and expands the use of AAV for gene delivery in vivo.
© 2023. The Author(s).
Conflict of interest statement
J.E.C., S.P. and A.S.P. are inventors on a patent filed by Stanford University regarding the use of AAVR to enhance and regulate AAV transduction. The other authors declare no competing interests.
Figures





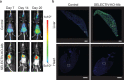
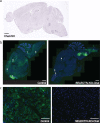
References
Publication types
MeSH terms
Grants and funding
- R01 AI158808/AI/NIAID NIH HHS/United States
- R01 AI153169/AI/NIAID NIH HHS/United States
- P30 EY026877/EY/NEI NIH HHS/United States
- R01 EY024665/EY/NEI NIH HHS/United States
- K99 NS120278/NS/NINDS NIH HHS/United States
- R01 EY030151/EY/NEI NIH HHS/United States
- R01 EB028646/EB/NIBIB NIH HHS/United States
- R01 AG069858/AG/NIA NIH HHS/United States
- R01 EY032159/EY/NEI NIH HHS/United States
- R01 AG020961/AG/NIA NIH HHS/United States
- R01 AI169467/AI/NIAID NIH HHS/United States
- R01 EY025295/EY/NEI NIH HHS/United States
- R00 NS120278/NS/NINDS NIH HHS/United States
- I01 CX001481/CX/CSRD VA/United States
- R01 AI141970/AI/NIAID NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- R01 EY025225/EY/NEI NIH HHS/United States
- R01 AI130123/AI/NIAID NIH HHS/United States
- R01 AI140186/AI/NIAID NIH HHS/United States
- R01 CA219994/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

