Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells
- PMID: 37291433
- PMCID: PMC10504080
- DOI: 10.1038/s41551-023-01050-0
Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells
Abstract
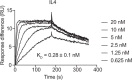
Immunoparalysis is a compensatory and persistent anti-inflammatory response to trauma, sepsis or another serious insult, which increases the risk of opportunistic infections, morbidity and mortality. Here, we show that in cultured primary human monocytes, interleukin-4 (IL4) inhibits acute inflammation, while simultaneously inducing a long-lasting innate immune memory named trained immunity. To take advantage of this paradoxical IL4 feature in vivo, we developed a fusion protein of apolipoprotein A1 (apoA1) and IL4, which integrates into a lipid nanoparticle. In mice and non-human primates, an intravenously injected apoA1-IL4-embedding nanoparticle targets myeloid-cell-rich haematopoietic organs, in particular, the spleen and bone marrow. We subsequently demonstrate that IL4 nanotherapy resolved immunoparalysis in mice with lipopolysaccharide-induced hyperinflammation, as well as in ex vivo human sepsis models and in experimental endotoxemia. Our findings support the translational development of nanoparticle formulations of apoA1-IL4 for the treatment of patients with sepsis at risk of immunoparalysis-induced complications.
© 2023. The Author(s).
Conflict of interest statement
W.J.M.M., L.A.B.J. and M.G.N. are scientific co-founders of and have equity in Trained Therapeutix Discovery. W.J.M.M. is CSO of Trained Therapeutix Discovery. W.J.M.M. and M.G.N. are scientific co-founders of and have equity in BioTrip.
Figures






References
-
- Bruse, N., Leijte, G. P., Pickkers, P. & Kox, M. in Expert Review of Clinical Immunology, Vol. 15, 251–263 (Taylor and Francis, 2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

