Antibiotics in the clinical pipeline as of December 2022
- PMID: 37291465
- PMCID: PMC10248350
- DOI: 10.1038/s41429-023-00629-8
Antibiotics in the clinical pipeline as of December 2022
Erratum in
-
Correction: Antibiotics in the clinical pipeline as of December 2022.J Antibiot (Tokyo). 2024 Jan;77(1):71. doi: 10.1038/s41429-023-00671-6. J Antibiot (Tokyo). 2024. PMID: 37935824 Free PMC article. No abstract available.
Abstract
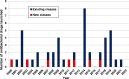
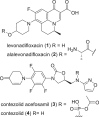
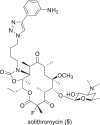
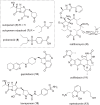
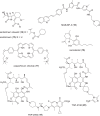
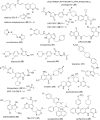
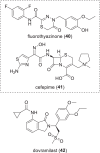
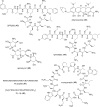
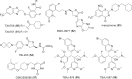
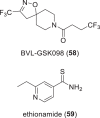
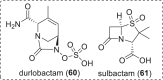
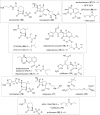
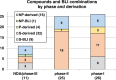
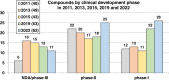
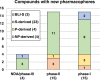
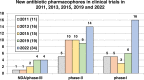
The need for new antibacterial drugs to treat the increasing global prevalence of drug-resistant bacterial infections has clearly attracted global attention, with a range of existing and upcoming funding, policy, and legislative initiatives designed to revive antibacterial R&D. It is essential to assess whether these programs are having any real-world impact and this review continues our systematic analyses that began in 2011. Direct-acting antibacterials (47), non-traditional small molecule antibacterials (5), and β-lactam/β-lactamase inhibitor combinations (10) under clinical development as of December 2022 are described, as are the three antibacterial drugs launched since 2020. Encouragingly, the increased number of early-stage clinical candidates observed in the 2019 review increased in 2022, although the number of first-time drug approvals from 2020 to 2022 was disappointingly low. It will be critical to monitor how many Phase-I and -II candidates move into Phase-III and beyond in the next few years. There was also an enhanced presence of novel antibacterial pharmacophores in early-stage trials, and at least 18 of the 26 phase-I candidates were targeted to treat Gram-negative bacteria infections. Despite the promising early-stage antibacterial pipeline, it is essential to maintain funding for antibacterial R&D and to ensure that plans to address late-stage pipeline issues succeed.
© 2023. The Author(s).
Conflict of interest statement
MATB has conducted antibiotic research that was funded by Botanix Pharmaceuticals and has received funding from CARB-X. MATB is an inventor of patents describing novel antibiotics.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

