Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials
- PMID: 37291608
- PMCID: PMC10249258
- DOI: 10.1186/s12943-023-01800-3
Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials
Erratum in
-
Correction: Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials.Mol Cancer. 2023 Jun 27;22(1):101. doi: 10.1186/s12943-023-01812-z. Mol Cancer. 2023. PMID: 37370104 Free PMC article. No abstract available.
Abstract
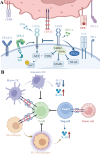
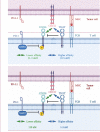
Over the past decade, immune checkpoint inhibitors (ICIs) have emerged as a revolutionary cancer treatment modality, offering long-lasting responses and survival benefits for a substantial number of cancer patients. However, the response rates to ICIs vary significantly among individuals and cancer types, with a notable proportion of patients exhibiting resistance or showing no response. Therefore, dual ICI combination therapy has been proposed as a potential strategy to address these challenges. One of the targets is TIGIT, an inhibitory receptor associated with T-cell exhaustion. TIGIT has diverse immunosuppressive effects on the cancer immunity cycle, including the inhibition of natural killer cell effector function, suppression of dendritic cell maturation, promotion of macrophage polarization to the M2 phenotype, and differentiation of T cells to regulatory T cells. Furthermore, TIGIT is linked with PD-1 expression, and it can synergize with PD-1/PD-L1 blockade to enhance tumor rejection. Preclinical studies have demonstrated the potential benefits of co-inhibition of TIGIT and PD-1/PD-L1 in enhancing anti-tumor immunity and improving treatment outcomes in several cancer types. Several clinical trials are underway to evaluate the safety and efficacy of TIGIT and PD-1/PD-L1 co-inhibition in various cancer types, and the results are awaited. This review provides an overview of the mechanisms of TIGIT and PD-1/PD-L1 co-inhibition in anti-tumor treatment, summarizes the latest clinical trials investigating this combination therapy, and discusses its prospects. Overall, co-inhibition of TIGIT and PD-1/PD-L1 represents a promising therapeutic approach for cancer treatment that has the potential to improve the outcomes of cancer patients treated with ICIs.
Keywords: Combined therapy; Immune checkpoint inhibitors; PD-1; PD-L1; TIGIT.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab Plus Ipilimumab or Placebo for metastatic non-small-cell lung Cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39:2327–38. doi: 10.1200/JCO.20.03579. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

