Co-trimoxazole prophylaxis for children who are HIV-exposed and uninfected: a systematic review
- PMID: 37292018
- PMCID: PMC10251133
- DOI: 10.1002/jia2.26079
Co-trimoxazole prophylaxis for children who are HIV-exposed and uninfected: a systematic review
Abstract
Introduction: Co-trimoxazole prophylaxis is recommended for children born to women with HIV to protect those who acquire HIV from opportunistic infections, severe bacterial infections and malaria. With scale-up of maternal antiretroviral therapy, most children remain HIV-exposed uninfected (HEU) and the benefits of universal co-trimoxazole are uncertain. We assessed the effect of co-trimoxazole on mortality and morbidity of children who are HEU.
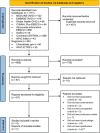
Methods: We performed a systematic review (PROSPERO number: CRD42021215059). We systematically searched MEDLINE, Embase, Cochrane CENTRAL, Global Health, CINAHL Plus, Africa-Wide Information, SciELO and WHO Global Index Medicus for peer-reviewed articles from inception to 4th January 2022 without limits. Ongoing randomized controlled trials (RCTs) were identified through registries. We included RCTs reporting mortality or morbidity in children who are HEU receiving co-trimoxazole versus no prophylaxis/placebo. The risk of bias was assessed using the Cochrane 2.0 tool. Data were summarized using narrative synthesis and findings were stratified by malaria endemicity.
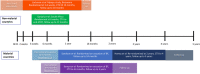
Results: We screened 1257 records and included seven reports from four RCTs. Two trials from Botswana and South Africa of 4067 children who are HEU found no difference in mortality or infectious morbidity in children randomized to co-trimoxazole prophylaxis started at 2-6 weeks of age compared to those randomized to placebo or no treatment, although event rates were low. Sub-studies found that antimicrobial resistance was higher in infants receiving co-trimoxazole. Two trials in Uganda investigating prolonged co-trimoxazole after breastfeeding cessation showed protection against malaria but no other morbidity or mortality differences. All trials had some concerns or a high risk of bias, which limited the certainty of evidence.
Discussion: Studies show no clinical benefit of co-trimoxazole prophylaxis in children who are HEU, except to prevent malaria. Potential harms were identified for co-trimoxazole prophylaxis leading to antimicrobial resistance. The trials in non-malarial regions were conducted in populations with low mortality potentially reducing generalizability to other settings.
Conclusions: In low-mortality settings with few HIV transmissions and well-performing early infant diagnosis and treatment programmes, universal co-trimoxazole may not be required.
Keywords: HIV exposure; co-trimoxazole; infants; morbidity; mortality; public health.
© 2023 World Health Organization; licensed by International AIDS Society. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
AJP declares paid participation on the Botnar Research Centre for Child Health independent external review board. He is a member of several DSMBs with no payment, none of which relate to the current research. All other authors declare no competing interests.
Figures

References
-
- UNAIDS . AIDSinfo. Available from: http://aidsinfo.unaids.org. Accessed 27th July 2021.
-
- Bourne DE, Thompson M, Brody LL, Cotton M, Draper B, Laubscher R, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23(1):101–6. - PubMed
-
- Church JA, Fitzgerald F, Walker AS, Gibb DM, Prendergast AJ. The expanding role of co‐trimoxazole in developing countries. Lancet Infect Dis. 2015;. 15(3):327–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

