Using light scattering to assess how phospholipid-protein interactions affect complex I functionality in liposomes
- PMID: 37292059
- PMCID: PMC10246558
- DOI: 10.1039/d2cb00158f
Using light scattering to assess how phospholipid-protein interactions affect complex I functionality in liposomes
Abstract
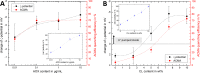
Complex I is an essential membrane protein in respiration, oxidising NADH and reducing ubiquinone to contribute to the proton-motive force that powers ATP synthesis. Liposomes provide an attractive platform to investigate complex I in a phospholipid membrane with the native hydrophobic ubiquinone substrate and proton transport across the membrane, but without convoluting contributions from other proteins present in the native mitochondrial inner membrane. Here, we use dynamic and electrophoretic light scattering techniques (DLS and ELS) to show how physical parameters, in particular the zeta potential (ζ-potential), correlate strongly with the biochemical functionality of complex I-containing proteoliposomes. We find that cardiolipin plays a crucial role in the reconstitution and functioning of complex I and that, as a highly charged lipid, it acts as a sensitive reporter on the biochemical competence of proteoliposomes in ELS measurements. We show that the change in ζ-potential between liposomes and proteoliposomes correlates linearly with protein retention and catalytic oxidoreduction activity of complex I. These correlations are dependent on the presence of cardiolipin, but are otherwise independent of the liposome lipid composition. Moreover, changes in the ζ-potential are sensitive to the proton motive force established upon proton pumping by complex I, thereby constituting a complementary technique to established biochemical assays. ELS measurements may thus serve as a more widely useful tool to investigate membrane proteins in lipid systems, especially those that contain charged lipids.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
- Stillwell W., in An Introduction to Biological Membranes, ed. W. Stillwell, Elsevier, 2016, pp. 3–15
-
- Rigaud J.-L. and Lévy D., Membrane Protein Reconstitution, Elsevier, 2003, vol. 372, pp. 65–86 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

