Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration
- PMID: 37292230
- PMCID: PMC10245070
- DOI: 10.1016/j.bioactmat.2023.05.013
Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration
Abstract
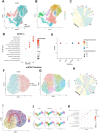
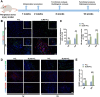
Biomaterials can modulate the local immune and repair-supportive microenvironments to promote peripheral nerve regeneration. Inorganic bioceramics have been widely used for regulating tissue regeneration and local immune response. However, little is known on whether inorganic bioceramics can have potential for enhancing peripheral nerve regeneration and what are the mechanisms underlying their actions. Here, the inorganic lithium-magnesium-silicon (Li-Mg-Si, LMS) bioceramics containing scaffolds are fabricated and characterized. The LMS-containing scaffolds had no cytotoxicity against rat Schwann cells (SCs), but promoted their migration and differentiation towards a remyelination state by up-regulating the expression of neurotrophic factors in a β-catenin-dependent manner. Furthermore, using single cell-sequencing, we showed that LMS-containing scaffolds promoted macrophage polarization towards the pro-regenerative M2-like cells, which subsequently facilitated the migration and differentiation of SCs. Moreover, implantation with the LMS-containing nerve guidance conduits (NGCs) increased the frequency of M2-like macrophage infiltration and enhanced nerve regeneration and motor functional recovery in a rat model of sciatic nerve injury. Collectively, these findings indicated that the inorganic LMS bioceramics offered a potential strategy for enhancing peripheral nerve regeneration by modulating the immune microenvironment and promoting SCs remyelination.
Keywords: Bioceramics; Immuno-modulation microenvironment; Macrophage; Nerve guidance conduit; Peripheral nerve regeneration.
© 2023 The Authors.
Conflict of interest statement
All the authors have no conflict of interests.
Figures








References
LinkOut - more resources
Full Text Sources

