This is a preprint.
The evolution of next-generation sequencing technologies
- PMID: 37292469
- PMCID: PMC10246072
The evolution of next-generation sequencing technologies
Update in
-
The Evolution of Next-Generation Sequencing Technologies.Methods Mol Biol. 2025;2866:3-29. doi: 10.1007/978-1-0716-4192-7_1. Methods Mol Biol. 2025. PMID: 39546194 Review.
Abstract
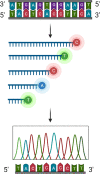
The genetic information that dictates the structure and function of all life forms is encoded in the DNA. In 1953, Watson and Crick first presented the double helical structure of a DNA molecule. Their findings unearthed the desire to elucidate the exact composition and sequence of DNA molecules. Discoveries and the subsequent development and optimization of techniques that allowed for deciphering the DNA sequence has opened new doors in research, biotech, and healthcare. The application of high-throughput sequencing technologies in these industries has positively impacted and will continue to contribute to the betterment of humanity and the global economy. Improvements, such as the use of radioactive molecules for DNA sequencing to the use of florescent dyes and the implementation of polymerase chain reaction (PCR) for amplification, led to sequencing a few hundred base pairs in days, to automation, where sequencing of thousands of base pairs in hours became possible. Significant advances have been made, but there is still room for improvement. Here, we look at the history and the technology of the currently available high-through put sequencing platforms and the possible applications of such technologies to biomedical research and beyond.
Keywords: DNA-seq; Next-generation sequencing; RNA-seq; high-throughput sequencing; single-molecule sequencing.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
