This is a preprint.
Bayesian target optimisation for high-precision holographic optogenetics
- PMID: 37292661
- PMCID: PMC10246014
- DOI: 10.1101/2023.05.25.542307
Bayesian target optimisation for high-precision holographic optogenetics
Abstract
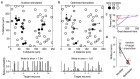
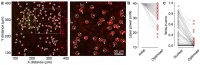
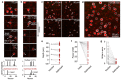
Two-photon optogenetics has transformed our ability to probe the structure and function of neural circuits. However, achieving precise optogenetic control of neural ensemble activity has remained fundamentally constrained by the problem of off-target stimulation (OTS): the inadvertent activation of nearby non-target neurons due to imperfect confinement of light onto target neurons. Here we propose a novel computational approach to this problem called Bayesian target optimisation. Our approach uses nonparametric Bayesian inference to model neural responses to optogenetic stimulation, and then optimises the laser powers and optical target locations needed to achieve a desired activity pattern with minimal OTS. We validate our approach in simulations and using data from in vitro experiments, showing that Bayesian target optimisation considerably reduces OTS across all conditions we test. Together, these results establish our ability to overcome OTS, enabling optogenetic stimulation with substantially improved precision.
Figures


References
-
- Prakash Rohit, Yizhar Ofer, Grewe Benjamin, Ramakrishnan Charu, Wang Nancy, Goshen Inbal, Packer Adam M, Peterka Darcy S, Yuste Rafael, Schnitzer Mark J, et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nature Methods, 9(12):1171–1179, 2012. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
