This is a preprint.
Caenorhabditis elegans germ granules are present in distinct configurations that differentially associate with RNAi-targeted RNAs
- PMID: 37292702
- PMCID: PMC10246010
- DOI: 10.1101/2023.05.25.542330
Caenorhabditis elegans germ granules are present in distinct configurations that differentially associate with RNAi-targeted RNAs
Update in
-
Caenorhabditis elegans germ granules are present in distinct configurations and assemble in a hierarchical manner.Development. 2023 Dec 15;150(24):dev202284. doi: 10.1242/dev.202284. Epub 2023 Dec 11. Development. 2023. PMID: 38009921 Free PMC article.
Abstract
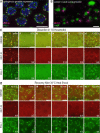
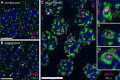
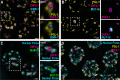
RNA silencing pathways are complex, highly conserved, and perform widespread, critical regulatory roles. In C. elegans germlines, RNA surveillance occurs through a series of perinuclear germ granule compartments-P granules, Z granules, SIMR foci, and Mutator foci-multiple of which form via phase separation and exhibit liquid-like properties. The functions of individual proteins within germ granules are well-studied, but the spatial organization, physical interaction, and coordination of biomolecule exchange between compartments within germ granule "nuage" is less understood. Here we find that key proteins are sufficient for compartment separation, and that the boundary between compartments can be reestablished after perturbation. Using super-resolution microscopy, we discover a toroidal P granule morphology which encircles the other germ granule compartments in a consistent exterior-to-interior spatial organization. Combined with findings that nuclear pores primarily interact with P granules, this nuage compartment organization has broad implications for the trajectory of an RNA as it exits the nucleus and enters small RNA pathway compartments. Furthermore, we quantify the stoichiometric relationships between germ granule compartments and RNA to reveal discrete populations of nuage that differentially associate with RNAi-targeted transcripts, possibly suggesting functional differences between nuage configurations. Together, our work creates a more spatially and compositionally accurate model of C. elegans nuage which informs the conceptualization of RNA silencing through different germ granule compartments.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing or financial interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
